Organoids – mimicking our organs in the lab
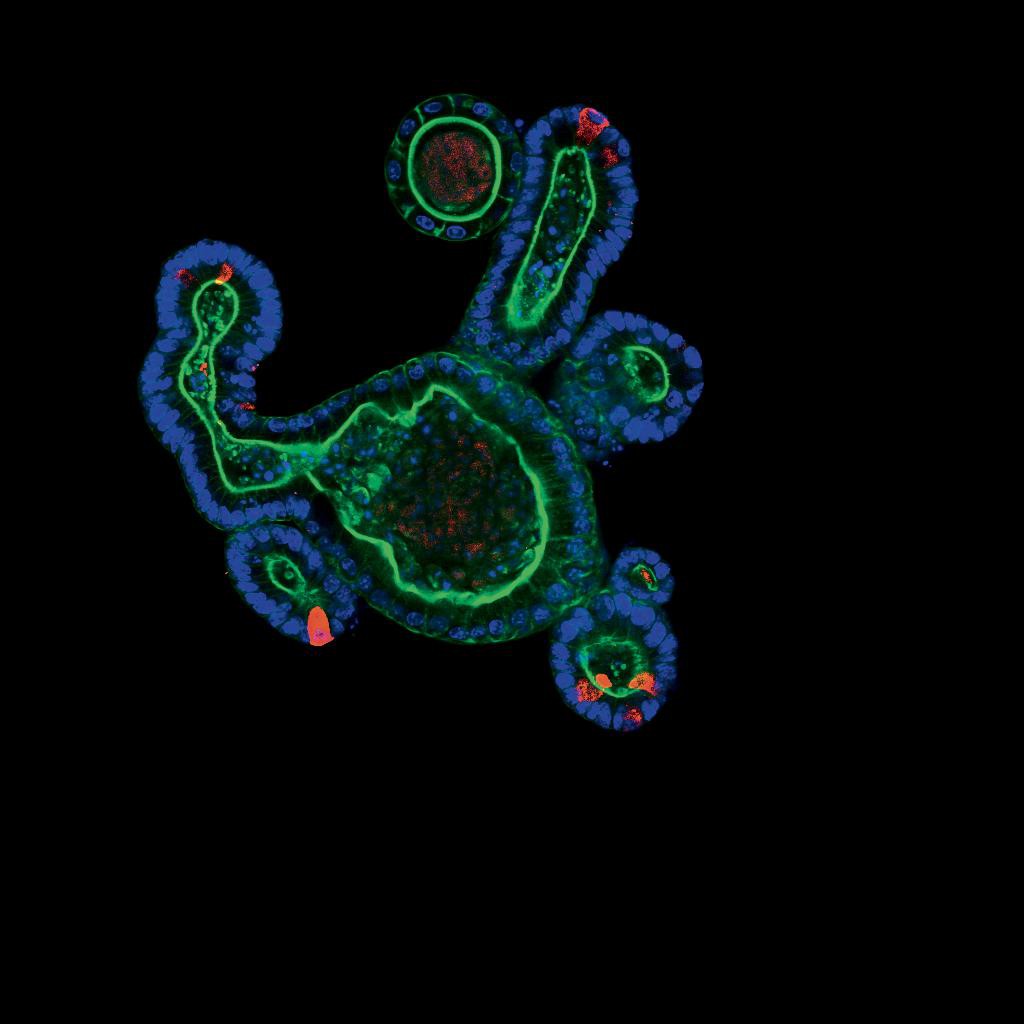
© Saba Rezakhani, Lütolf lab/EPFL
ORGANOIDS FOLDER 1/3 – Organoids, which are miniaturized organs no bigger than a pepper seed, have been revolutionizing stem-cell research for the past ten years, bridging the gap between in vitro and in vivo. They offer unparalleled prospects for personalized medicine, cancer treatment and disease modeling. EPFL has just joined forces with Lausanne University Hospital (CHUV) for a new study involving this technology, which has already given rise to several startups on the EPFL campus.
It was just under ten years ago that a major new development shook the very foundations of biology. In 2009, Hans Clevers’ laboratory at the Hubrecht Institute in the Netherlands published a major paper revealing for the first time a method for growing an organoid of an intestine. “It was a real shock for a lot of biologists,” recalls Matthias Lütolf, whose EPFL lab specializes in stem-cell biology and bioengineering. “This discovery went against the premise that, to become a tissue, a cell needed to be part of an organism and communicate with many other types of cells.” The study very clearly showed that stem cells could self-organize and adopt tissue and organ functions if they were cultured in a suitable three-dimensional environment. Europe was a pioneer and remains at the forefront of this research. And EPFL’s rich engineering environment makes it particularly attractive to biologists from around the world who are looking to enhance this technology.
Personalized medicine
Organoids have helped bridge the gap between in vitro and in vivo. These increasingly realistic, three-dimensional cell cultures are paving the way for new research into diagnostics, drug treatments and disease modeling, and can even be used to replace damaged tissue. Personalized medicine will also benefit, particularly when it comes to treating cancer. Organoids have the ability to express the same mutations as the original cells taken from the diseased tissue of a patient. They can reproduce an anomaly that is genetically unique to a given individual. A three-year partnership between EPFL and CHUV’s oncology department has just begun, with the aim of developing ways of predicting how patients will respond to cancer treatments. “When cultured ex vivo, organoids don’t develop in exactly the same way as the cells in a human body,” explains Dr. Krisztian Homicsko. “To resolve this, we need to optimize the cultures in which the cells grow. By working with Matthias Lütolf’s lab, we can for the first time analyze and compare our samples with their organoids.” This is such a promising field that the medical world has started to create banks of organoids to test drugs in a similar way to a clinical trial.
Ethics and animal testing
The role of ethics in cell culture is a hot topic – the journal Nature recently dedicated two pages to this question. This is because organoids could become micro hearts or brains with all the features of embryonic brain tissue. If it’s possible to mimic the physiological characteristics of a human brain, will it be possible to surveil that person as well? And will animal testing still be needed? “Let’s not get ahead of ourselves, a cell culture is a long way from a test on a living animal,” says Denis Duboule, whose specialties are embryology, genetics and the genomics of mammal development. “Organoids may reduce the need for animal testing, but they won’t replace it altogether. It won’t be possible to test all research topics on organoids, and study results will still have to be verified on an animal.” Duboule’s lab has even managed to create organoids of early embryos.
Just like genome medicine back in the day, organoids will soon be able to provide answers to a number of scientific questions.

Micro organs
Researchers have cultured organoids of the brain, thyroid, thymus, intestine, liver, pancreas, stomach, lungs, kidneys and early embryos. But how do they create these miniature organs?
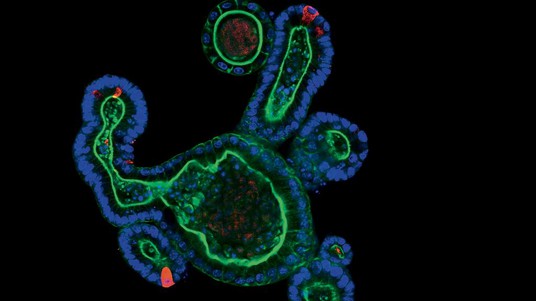
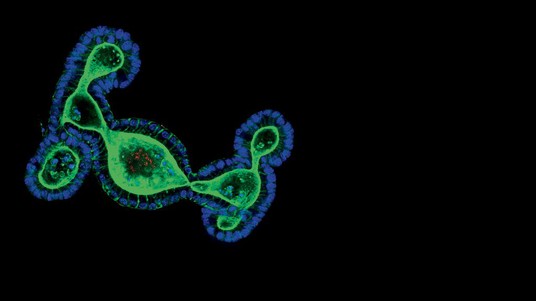
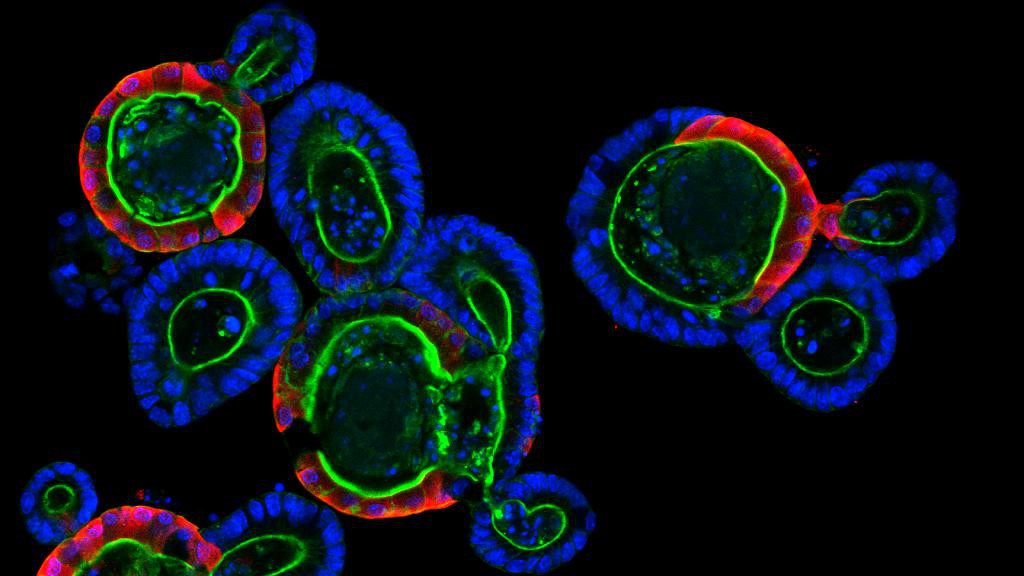
Our organs are made up of different types of cells that are organized in layers or in a network. It’s not a matter of simply putting the stem cells in a Petri dish and waiting for an organoid to grow. In order to create the three-dimensional tissue, researchers first have to identify, decode and understand the molecular signals that allow the stem cells to differentiate in the embryo or adult tissue. To self-organize and reproduce what they do naturally during embryogenesis – i.e., adopt the functionalities of tissue and start building an organ – stem cells need to be surrounded by the right cocktail of signals and a three-dimensional culture system that closely resembles the physiological conditions in vivo. In addition to stem cells, organoids, which typically measure between 300 and 500 microns (0.3–0.5 millimeters), can also be created indirectly from differentiated adult cells – those cells that have already been programmed within an organ. Researchers have managed to make stem cells “pluripotent,” which means they have the potential to differentiate into any type of cell in the organism. Researchers can therefore use organoids to gain greater insight into how our organs grow ex vivo.
Find all the articles of the folder dedicated to organoids:
1 - Organoids – mimicking our organs in the lab