Unraveling the initial molecular events of respiration
Hemoglobin. Credit: iStock photos/theasis
Physicists from Switzerland, Japan and Germany have unveiled the mechanism by which the first event of respiration takes place in heme proteins.
Respiration is a fundamental process of all living things, allowing them to produce energy, stay healthy, and survive. In cells, respiration involves what are known as “respiratory proteins”, e.g. hemoglobin in the blood and myoglobin in muscles.
Respiratory proteins work by binding and releasing small molecules like oxygen, carbon monoxide etc., called ligands. They do this through their “active center”, which in many respiratory proteins is a chemical structure called heme porphyrin.
Binding and releasing small molecules causes changes in the heme’s molecular and electronic structure. Such a change is the transition from a planar low spin ligated porphyrin form to a domed high spin un-ligated form and vice-versa. This shift is a key step for respiration, ultimately switching hemoglobin between a “relaxed” and “tense” conformation.
Electrons spin around atoms, but also spin around themselves, and can cross over from one spin state to another. The debate about the transition from low-spin planar to a high-spin domed heme has been dominated by two schools of thought: the process is either by thermal relaxation or by a cascade among electron spin states.
Now, a team of scientists led by Majed Chergui at EPFL’s School of Basic Sciences have solved the debate. The researchers detached the small molecule from the heme using short, energizing laser pulses. They then used another short, hard X-ray pulse from an X-ray free-electron laser to induce X-ray emission (XES), a very sensitive fingerprint of the spin state of molecules, which monitored the heme’s changes as a function of time. They could thus determine that the passage from planar to domed and back is caused by a cascade among spin states.
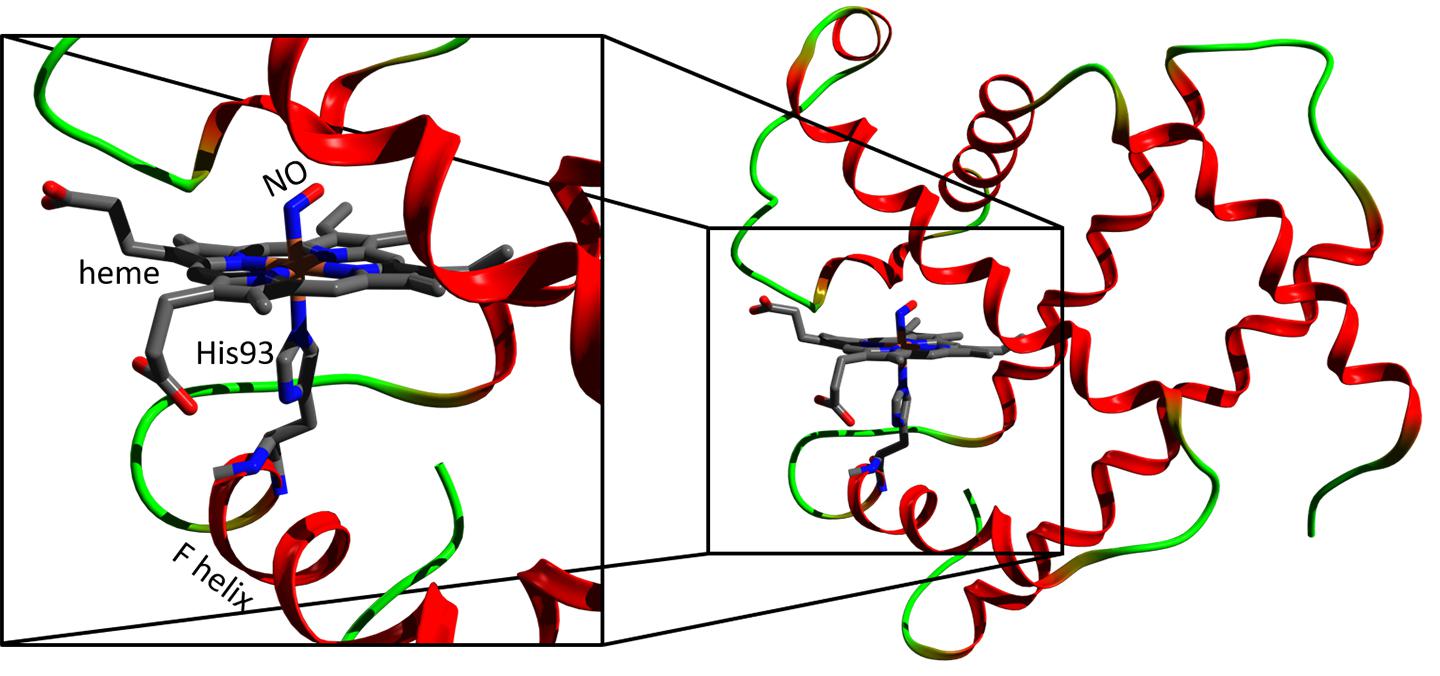
Myoglobin (right) and its heme region (left). Credit: Majed Chergui (EPFL)
The study was carried out on nitrosyl-myoglobin, which is myoglobin that has bound a nitric oxide molecule. Nitrosyl-myoglobin plays a crucial role in neurotransmission, regulation of vasodilatation, platelet aggregation, and immune responses.
“The conclusions of our work apply to all heme proteins,” says Chergui. “In particular to hemoglobin in its uptake and release of oxygen when we breathe. Although this takes place at the thermal temperatures of the body, breathing is governed by electronic changes in the heme.”
Other contributors
- Japan Synchrotron Radiation Research Institute (JASRI)
- University of Agriculture and Technology (TUAT), Japan
- Kyoto University
- Sofia University, Japan
- Newcastle University
- European XFEL, Germany
- Adam Mickiewicz University, Poland
- Paul-Scherrer-Institut (PSI), Switzerland
Swiss National Science foundation (NCCR:MUST) European Research Council (H2020) Inter-MUST Women Postdoc Fellowship Leverhulme Trust European XFEL Polish National Science Centre
Dominik Kinschel, Camila Bacellar, Oliviero Cannelli, Boris Sorokin, Tetsuo Katayama, Giulia F. Mancini, Jeremy R. Rouxel, Yuki Obara, Junichi Nishitani, Hironori Ito, Terumasa Ito, Naoya Kurahashi, Chika Higashimura, Shotaro Kudo, Theo Keane, Frederico A. Lima, Wojciech Gawelda, Peter Zalden, Sebastian Schulz, James M. Budarz, Dmitry Khakhulin, Andreas Galler, Christian Bressler, Christopher J. Milne, Thomas Penfold, Makina Yabashi, Toshinori Suzuki, Kazuhiko Misawa & Majed Chergui. Femtosecond X-ray emission study of the spin cross-over dynamics in heme proteins. Nature Communications DOI: 10.1038/s41467-020-17923-w