Understanding the defects that make water liquid
© 2016 EPFL
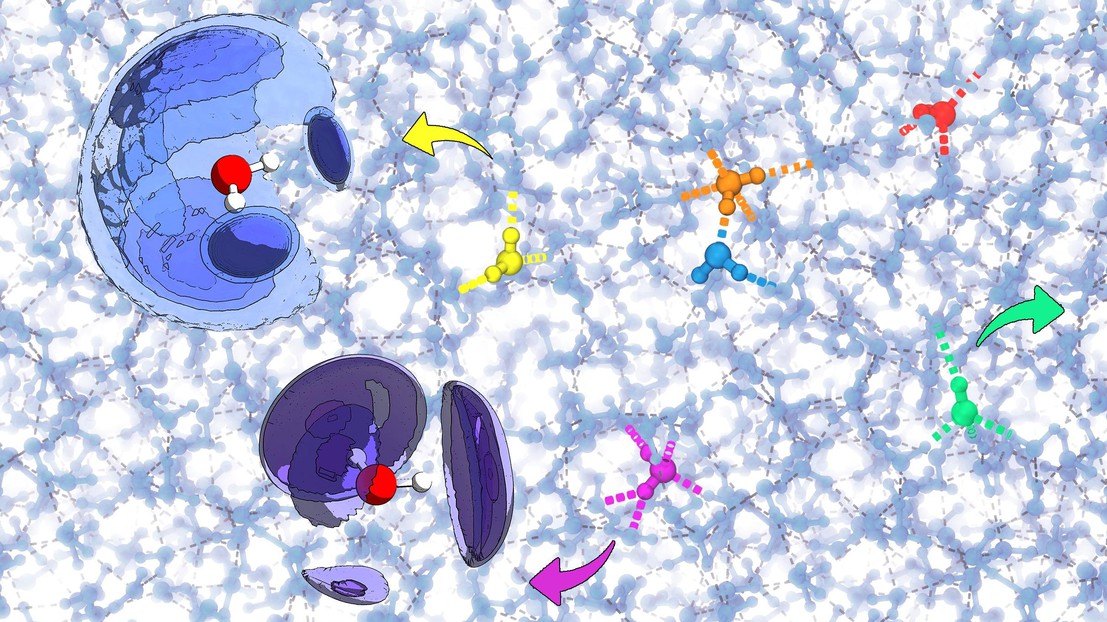
Water is characterized by a highly ordered hydrogen-bond network, which however contains many defects that make such network flexible enough to flow. Using machine-learning techniques it is possible to rationalize the relation between the geometric correlations among defects and their topology.
"Probing Defects and Correlations in the Hydrogen-Bond Network of ab Initio Water", selected for the cover of April's issue of the Journal of Chemical Theory and Computation, presents an estensive set of simulations of room-temperature liquid water, performed with both empirical and first-principles models of the inter-atomic forces, which are then analyzed based on machine-learning techniques.
The most common defects in the hydrogen-bond network of liquid water are identified, and the relationship between them is made clear through the use of three-dimensional correlation maps. It is found that the defects that are present in the liquid bear striking similarity to bonding patterns that are present in condensed phases of water, making it possible to rationalize some of the experimental probes of the structure of water.