Under Pressure: Petroleum Changes in the Deep Sea
Credit: J. Samuel Arey and Jonas Gros
Press Release: April 29, 2016
After the Deepwater Horizon accident in 2010, an often-asked question was: What happened to petroleum liquid and gas that was emitted into the deep sea, and why? A paper published recently by a team at the Ecole Polytechnique Fédérale de Lausanne, the Swiss Federal Institute of Aquatic Science and Technology (Eawag), Woods Hole Oceanographic Institution, and Texas A&M University sheds new light on the strange behaviors of petroleum liquid and natural gas in the deep ocean.
Unlike a more typical oil spill at the sea surface, a deep-sea petroleum release is affected by extreme high-pressure conditions, which fundamentally alter the state and behaviors of the petroleum in the sea environment. This is what happened during the 2010 Deepwater Horizon accident, when more than half a million tons of petroleum liquid and natural gas were released from the broken Macondo well at a depth of 1524 meters under water in the Gulf of Mexico, about 66 kilometers offshore from the Louisiana coast.
Extreme pressures
At a water depth of 1524 meters, the ambient pressure is extremely high, equivalent to the pressure felt inside a tennis ball that is being squashed under the weight of an African elephant.
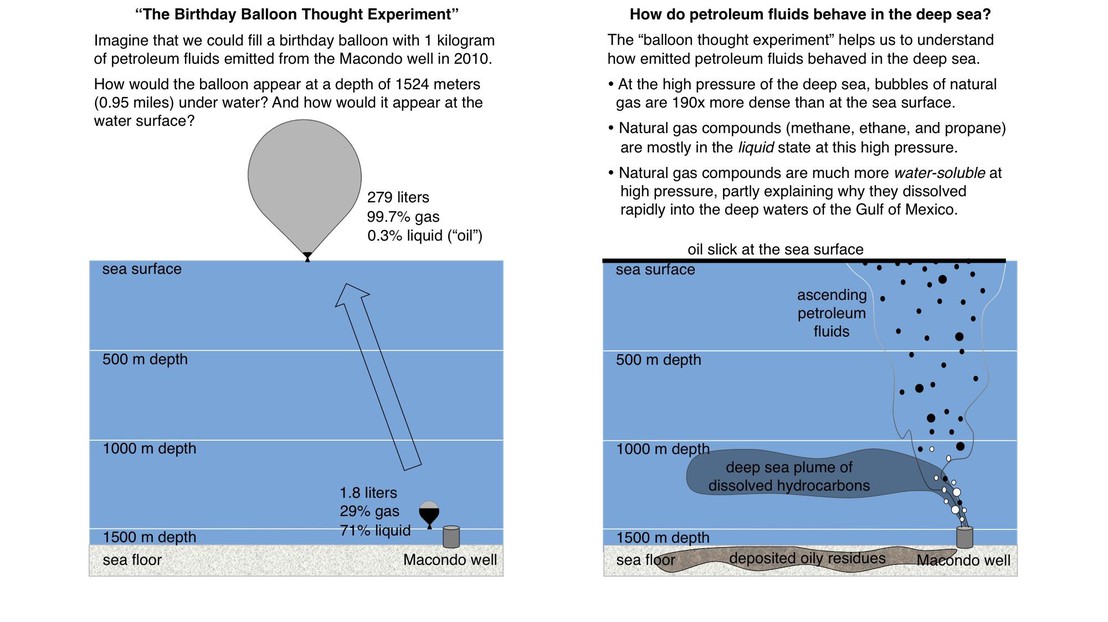
According to another thought experiment: if we would fill a birthday balloon with 1 kilogram of emitted petroleum fluids under the high pressure at 1524 meters under water, the balloon would have a volume of 1.8 liters, or about a half-gallon. However if the balloon was brought to the sea surface, it would expand to 279 liters, about the size of a household dishwasher.
"Liquified" natural gas
At the “low” pressures of the Earth's surface, methane, ethane, and propane are typically thought of as gases. However, at high pressure, these gases condense into liquid. At 1524 m depth, about 80% of the emitted propane would be in the liquid state, similar to the way that a high-pressure tank of a propane-fueled barbeque grill contains mostly liquid propane. Similarly, the petroleum fluids emitted into the deep sea were mostly in the liquid state.
Super-dense gas bubbles
Under this deep-sea pressure, the natural gas is about 190× more dense than natural gas at the sea surface. When pressure is increased, a larger number of gas molecules can be squeezed into the same volume of space, which increases the gas density. As a result, gas bubbles in the deep sea look and act like normal bubbles that you might see in a swimming pool, but they carry much more mass.
Natural gas dissolved in water
Deep-sea pressures also help natural gas compounds to dissolve into water. Methane, ethane, and propane would be 141×, 33×, and 11× more water-soluble at 1524 m depth than at the sea surface, largely due to the high pressure of the deep sea. This partly explains why emitted natural gas compounds such as methane, ethane, and propane never bubbled up to the sea surface. Instead, they dissolved rapidly and formed a deep-sea plume of dissolved hydrocarbons.
The rapid dissolution of natural gas in the deep sea was important. For example, this favored the formation of gas hydrates, which are crystal-like solid materials that arise from light hydrocarbon compounds and water under very high pressures. Gas hydrates obstructed efforts by response teams to stop the flow of petroleum from the severed Macondo well, which in turn prolonged the petroleum release, thereby leading to the largest accidental marine oil spill in recorded history.
Oily residues on the seafloor
Finally, the prolonged exposure to water and air probably contributed to the previously documented deposition of tens of thousands of tons of oil residues on the deep sea floor of the Gulf of Mexico. Typically, oil floats, because it is less dense than water. However, computer simulations show that the density of liquid petroleum progressively increases as light hydrocarbon compounds dissolve into the water column and evaporate into the atmosphere. Liquid petroleum thus becomes heavier with time in the sea environment. These oily residues may subsequently lose buoyancy entirely when they stick to dense solid particles such as dust particles or dead algae. Taken together, these and other processes make floating oil sink, thereby carrying petroleum residues down to the sea floor. This illustrates how a deep-sea petroleum release can affect marine life in the deep sea and on the sea floor, not only life at the coast.
This work was supported by the Gulf of Mexico Research Initiative (GOMRI), the DEEP-C consortium, and the Center of Integrated Modeling and Analysis of the Gulf Ecosystem (C-IMAGE).
Contact:
J. Samuel Arey
[email protected]