Towards cheaper hydrogen production via alkaline water splitting
© K.Sivula/2020 EPFL
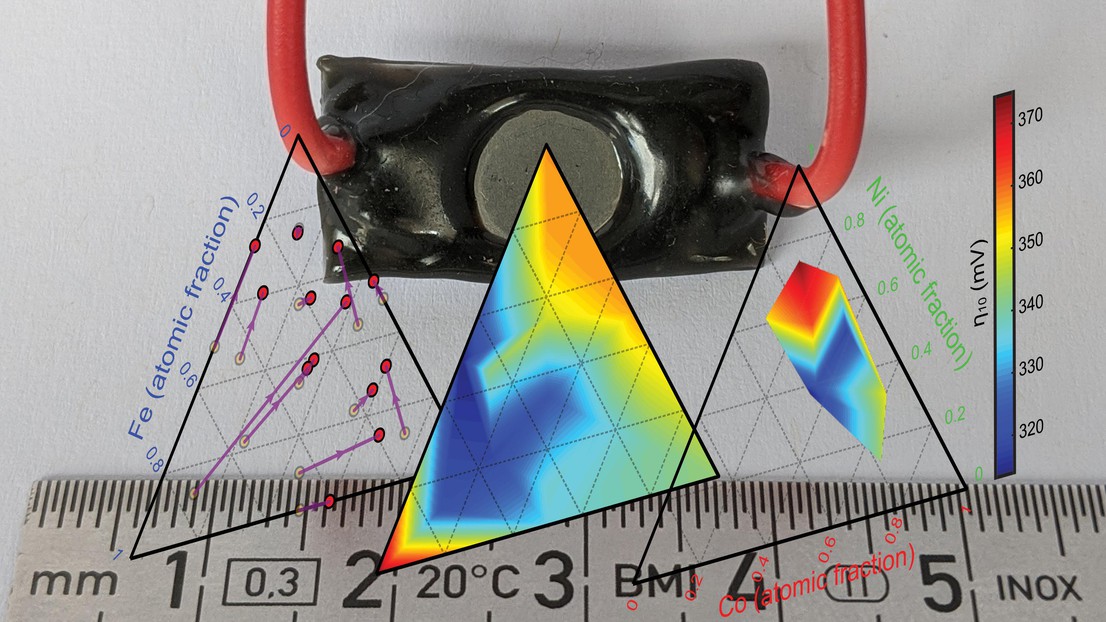
The ISIC laboratories of LIMNO and LMRE team up to uncover the influence of composition on performance in metallic Iron-Nickel-Cobalt ternary anodes for alkaline water electrolysis.
Metallic electrodes based on iron, nickel, and/or cobalt have re-emerged as promising cost-effective anodes for the alkaline oxygen evolution reaction (OER) due to their simplicity and their in-situ formation of a highly active oxy-hydroxide surface catalyst layer, which exhibits state-of-the-art overpotentials for the OER. However, the effect of alloy composition has not been systematically studied. In a new paper published in ACS Catalysis, using metallic anodes with defined Fe-Ni-Co atomic ratios prepared via arc melting in the LMRE lab and studied in the LIMNO lab, the paper report the relationship between the initially alloy composition, the OER performance, and the emergent active catalyst composition. Overall these results help to define the optimum Fe-Co-Ni metallic alloy anode composition for use in low-cost alkaline electrolysis.