The STING of death in T cells
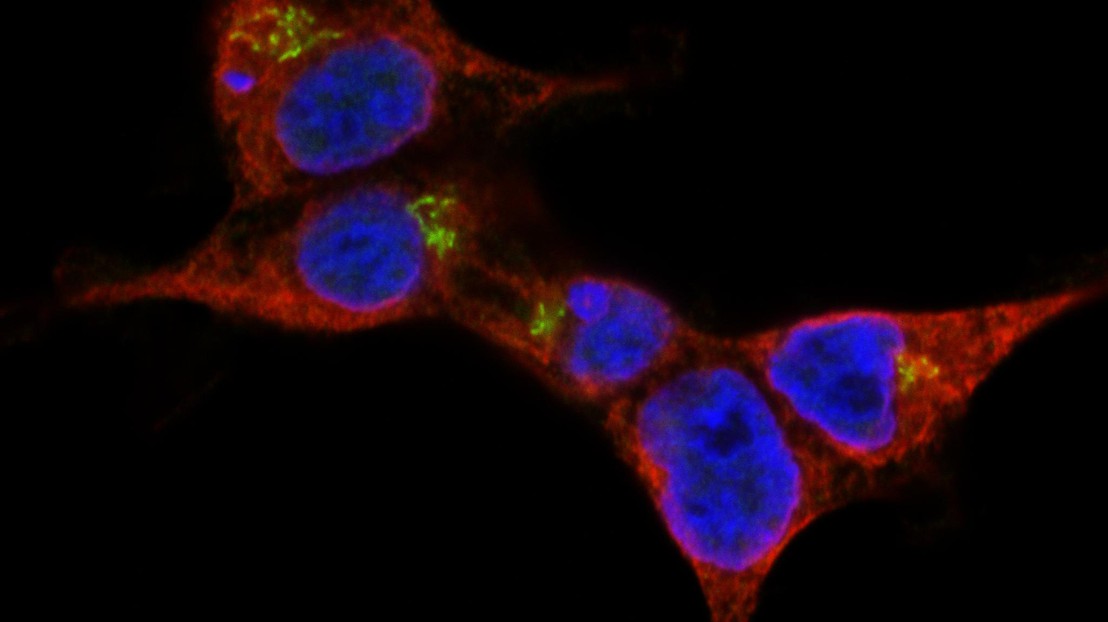
STING (green), a membrane protein located on the endoplasmic reticulum (red), rearranges upon activation and triggers cell type-specific death response. Credit: Simone Haag/A. Ablasser/EPFL
EPFL scientists show that the STING signaling pathway, which helps coordinate the innate immune system, causes cell death in T cells of the adaptive immune system. This “killing” effect includes cancerous T cells, and has implications for treating T cell-derived cancers.
The cells of the innate immune system use a signaling pathway comprising STING (Stimulator of interferon genes) to detect DNA from invading viruses and fight them. However, it is unknown if STING triggers the same or different responses in cells of the adaptive immune system, such as T cells. EPFL scientists have now shown that T cells have an “unconventional” STING response, which manifests as apoptotic cell death. The work, which may have implications for the treatment T cell-derived malignancies, is published in Nature Communications.
Innate immune system
The innate immune system is our first line of defense, made up of cells that quickly identify pathogens such as DNA from viruses. To do this, these cells use receptors that can identify nucleic acids — the building blocks of DNA — that in turn activate a signaling molecule called STING.
Once activated, the STING pathway turns on a set of genes that produce signaling molecules (cytokines) that help cells communicate with each other, as well as other cell-activating processes that fight off the infecting pathogen. But what we don’t know whether the STING response produces different outcomes between different cell types.
The STING of death
The lab of Andrea Ablasser at EPFL looked at the consequences of the STING pathway in T cells. Their analysis found that STING triggers the expression of BH3-only proteins — well known to be involved in cell death — which induces apoptosis in cells, as opposed to the production of cytokines such as interferons, which stimulate immune responses.
Interestingly, the researchers also found that this pro-apoptotic effect exists in cancerous T cells, such as the ones that cause T-cell lymphomas, which account for about a tenth of non-Hodgkin lymphomas. The EPFL scientists found that delivering a small molecule that activates the STING pathway prevents the growth of T cell-derived tumors in live animals.
The work shows an unanticipated connection between the magnitude of STING signaling and its ability to elicit different responses. This may allow for cell-type-adjusted behaviors in the presence of internal or external insults such as infections or oxidative stress.
The study uncovers a novel, non-immune effect of the STING pathway whereby it induces a different effect in cells of the adaptive immune system (T cells) than its effect in cells of the innate immune system. The fact that this effect is pro-apoptotic and is maintained even in cancerous T cells in vivo open up significant possibilities for treating T-cell lymphomas in the future.
Other contributing institutions
ISREC Foundation
Medical University of Innsbruck
INSERM UMR866
Tyrolean Cancer Research Institute
Funding
Swiss National Science Foundation
Novartis Foundation for medical-biological Research
Else Kröner Fresenius-Stiftung
Reference
Muhammet F. Gulen, Ute Koch, Simone M. Haag, Fabian Schuler, Lionel Apetoh, Andreas Villunger, Freddy Radtke, Andrea Ablasser. Signalling strength determines proapoptotic functions of STING.Nature Communications 05 September 2017. DOI: 10.1038/s41467-017-00573-w