The gene behind follicular lymphoma
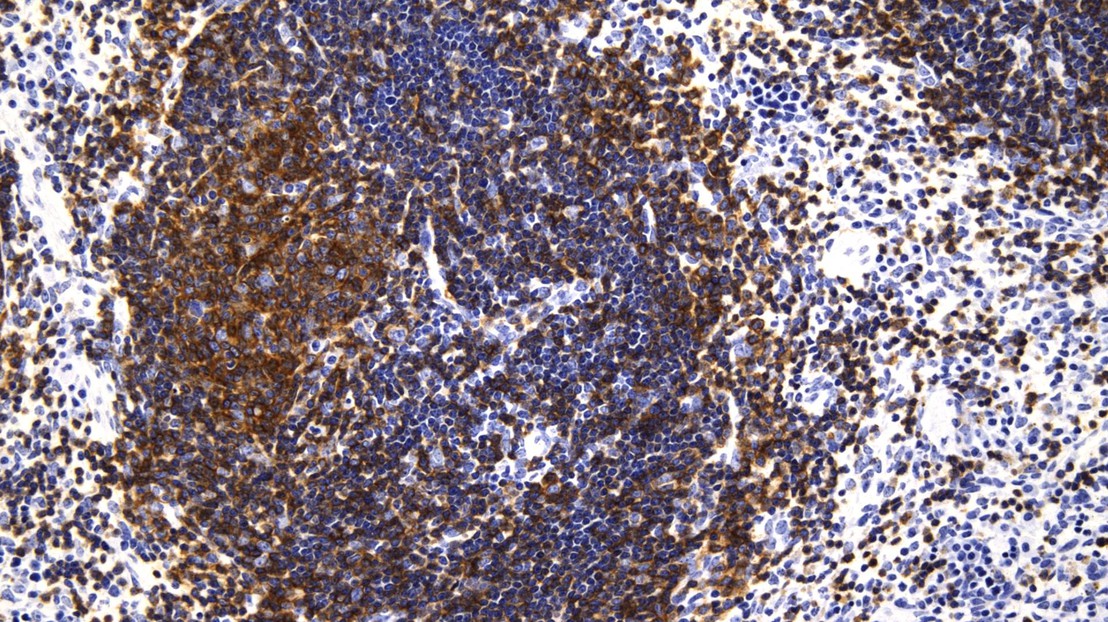
Expansion and accumulation of lymphoma cells (colored in brown) in mice spleen. Credit: Natalya Katanayeva (EPFL)
EPFL scientists have discovered an important gene whose loss lies behind follicular lymphoma, an incurable cancer.
Follicular lymphoma is an incurable cancer that affects over 200,000 people worldwide every year. A form of non-Hodgkin lymphoma, follicular lymphoma develops when the body starts making abnormal B-cells, which are white blood cells that in normal conditions fight infections. This cancer is associated with several alterations of the cell’s DNA, but it has been unclear which gene or genes are involved in its development. EPFL scientists have now analyzed the genomes of more than 200 patients with follicular lymphoma, and they discover that a gene, Sestrin1, is frequently missing or malfunctioning in follicular lymphoma patients. The discovery opens to new treatment options and it is now published in Science Translational Medicine.
One of the common features of follicular lymphoma is a genetic abnormality between two chromosomes (14 and 18). In an event known as “chromosomal translocation” the two chromosomes “swap” certain parts with each other. This triggers the activation of a gene that protects cells from dying, making cells virtually immortal — the hallmark of a tumor.
Moreover, approximately 30% of follicular lymphoma patients lose also a portion of chromosome 6, affecting multiple genes involved in suppressing the emergence of a tumor. These patients typically have poor prognosis. Another 20% of patients have alterations causing chromosomal disorganization and the consequent malfunctioning of several genes and proteins. The bottom line is that for both group of patients it is very difficult to pinpoint which of all the affected genes are actually causing the disease.
The lab of Elisa Oricchio at EPFL, with colleagues from the US and Canada, analyzed the genomes of over 200 follicular lymphoma patients. Their analyses revealed that a specific gene, Sestrin1, can be harmed by both loss of chromosome 6 and silenced in patients.
Sestrin1 helps the cell defending itself against DNA damage — for example after exposure to radiation — and oxidative stress. In fact, Sestrin1 is part of the cell’s anti-tumor mechanism that stops potentially cancerous cells from growing.
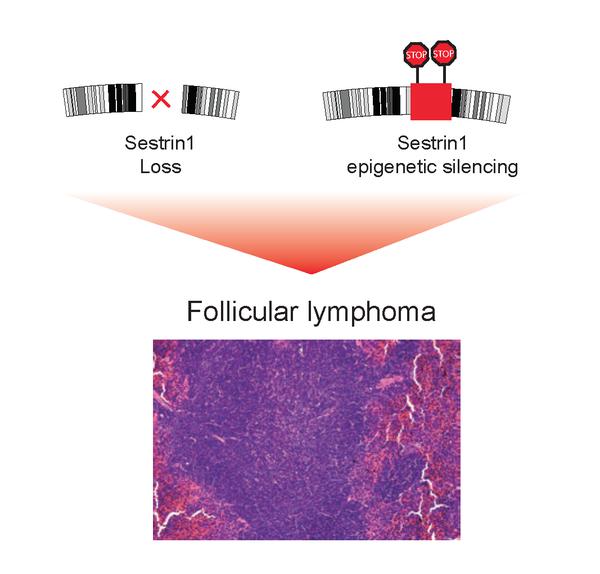
Disruption of a region in chromosome 6 or epigenetic modifications of the DNA block Sestrin1 expression and these contribute to the development of Follicular Lymphoma.
Beyond identifying the Sestrin1 gene as frequently altered in follicular lymphoma patients, the scientists demonstrated that Sestrin1 is able to suppress tumors in vivo. They showed that Sestrin1 exerts its anti-tumor effects by blocking the activity of a protein complex called mTORC1, which is well known for controlling protein synthesis as well as acting as a sensor for nutrient or energy changes in the cell.
Finally, the identification of loss of Sestrin1 as a key event behind the development of follicular lymphoma is particular important because it helps identifying patients that will benefit from new therapies. Indeed, this study shows that the therapeutic efficacy of a new drug that is currently in clinical trial depends on Sestrin1. Importantly, this dependency can be extended beyond follicular lymphoma to other tumor types.
This work was carried out in collaboration with the Memorial Sloan Kettering Cancer Center (New York), Cornell University, the University of Lausanne, Goodwin Research Laboratories, Trinity College Dublin, the BC Cancer Agency, the University of British Columbia, and the Princess Margaret Cancer Centre (Toronto).
Funding
Swiss National Science Foundation, ISREC Foundation, the Giorgi-Cavaglieri Foundation, the National Cancer Institute, the Lymphoma Research Foundation, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Memorial Sloan Kettering Cancer Center, the National Institutes of Health, the Starr Cancer Consortium, the Geoffrey Beene Cancer Research Center, the Leukemia and Lymphoma Society, and the Princess Margaret Cancer Centre.
Reference
E. Oricchio, N. Katanayeva, M. C. Donaldson, S. Sungalee, P. P. Joyce, W. Béguelin, E. Battistello, V. R. Sanghvi, M. Jiang, Y. Jiang, M. Teater, A. Parmigiani, A. V. Budanov, F. C. Chan, S. P. Shah, R. Kridel, A. M. Melnick, G. Ciriello, H-G. Wendel. Genetic and epigenetic inactivation of SESTRIN1 controls mTORC1 and response to EZH2 inhibition in follicular lymphoma.Science Translational Medicine 9, eaak9969 (2017). DOI: 10.1126/scitranslmed.aak9969