The Flexible Linker Strikes Again - in a new paper from LIMNO
© 2017 K.Sivula / EPFL
Xavier Jeanbourquin et al. compare two linking strategies for solution processed small molecule semiconductors, and discover greatly different optoelectronic properties. The work is published today in J. Mater. Chem. A.
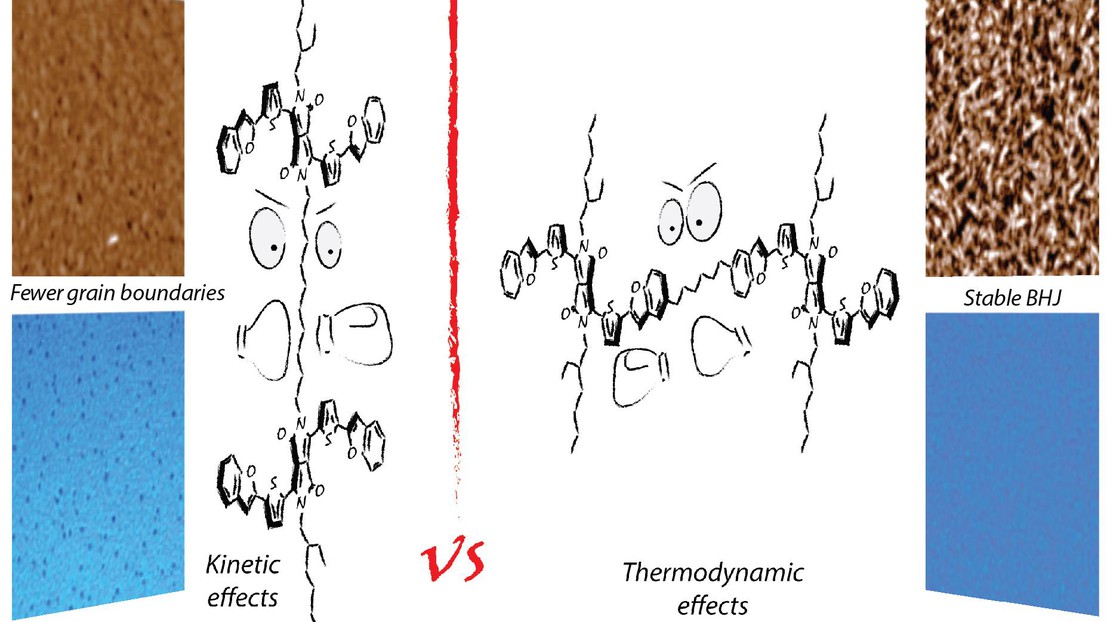
The solid-state self-assembly of molecular semiconductors is a key aspect for controlling the optoelectronic properties of organic electronic materials. In this work, we investigated the use of a flexible linker strategy to control the self-assembly of a solution-processable diketopyrrolopyrrole semiconductor coded as DPP(TBFu)2. Two distinct dimers—prepared with varied linker position relative to the orientation of the conjugated core—reveal the effect of connectivity on the solid-state self-assembly and optoelectronic properties—favoring either H- or J-type aggregation. The dimer with a “vertical” linker orientation exhibits a poor crystallinity in neat films, but improves hole mobility in OFETs 10-fold, reaching 3.0 × 10–3 cm2V–1s–1 when used as an additive in with DPP(TBFu)2. Distinctively, the dimer with a “horizontal” linking orientation does not enhance charge carrier transport, but is found to affect the thermal stability of donor:acceptor blends in OPVs with PCBM. Devices retain 90% of their initial conversion efficiency after 5 hours of thermal stress, compared to only 45% for control devices. Thermodynamic and kinetic rationale further suggest that this flexible linker strategy represents a powerful tool to control supramolecular assembly in molecular semiconductors without altering the nature of the core conjugated segment.