Structure and Mechanism of the Influenza A M218–60 Dimer of Dimers
© 2015 EPFL
On the cover of JACS. In a collaboration between MIT, ENS Lyon, Harvard Medical School and EPFL
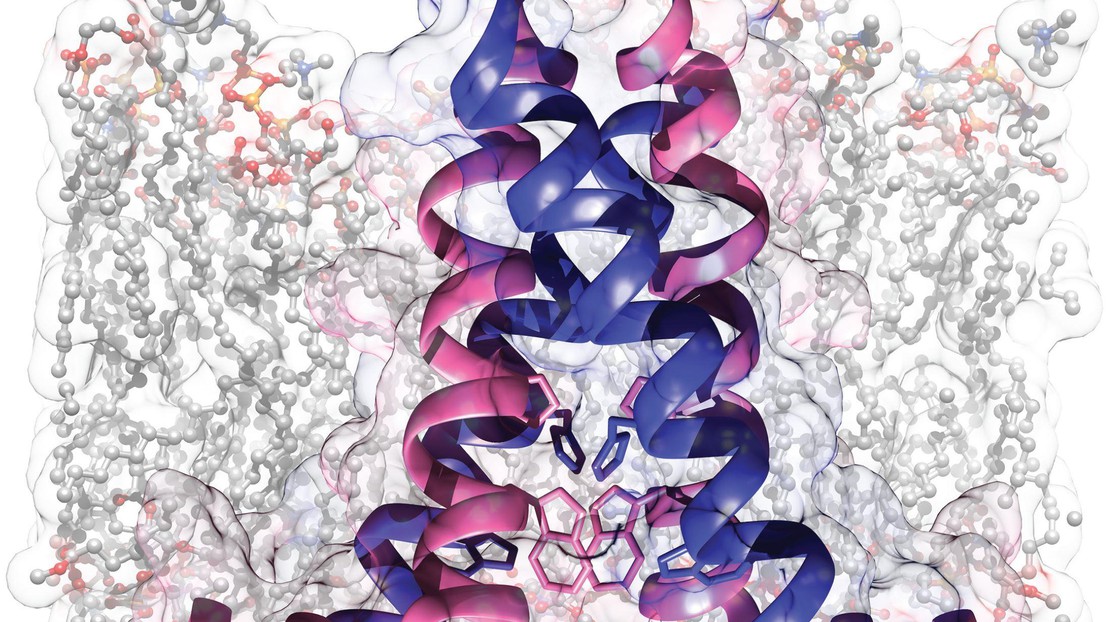
State of the art magic angle spinning NMR was used to determine the structure of the S31N mutant of the M2 membrane protein from influenza A. Influenza A M2 is a 97 residue transmembrane protein that acts as a H+ transporter and which is critical for viral replication, as evidenced by the therapeutic effect of aminoadamantyl inhibitors known to target M2 and to reduce proton conduction. However, resistance has developed in circulating strains of influenza, primarily due to a single-point mutation S31N, which has precipitated a need for new inhibitors that target S31N M2. Here the NMR structure shows that in phospholipid bilayers this M2 construct assembles as a dimer of dimers, rather than a tetramer as previously thought. Present are His–Trp contacts that are likely important in H+ conduction and steric constraints that explain the drug resistance of this mutant.”