STORM reveals the secrets of telomeres
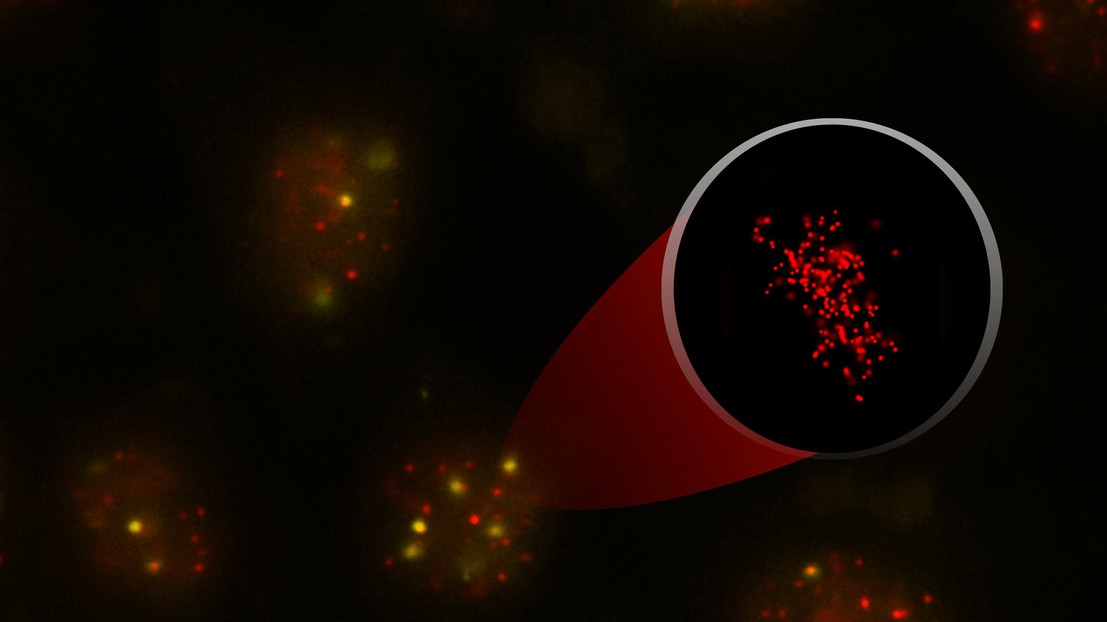
Interphase HeLa cells with their telomeres (red) and a protein marking sites of DNA damage (yellow). Insert: super-resolution of a telomere. Credit: K. M. Douglass/ A. Vancevska/J. Lingner (EPFL)
Using super-resolution microscopy, EPFL scientists have carried out an extensive study of damaged telomeres, which links to cancer and ageing.
Cells defend themselves from destructive DNA damage with a network of mechanisms collectively named the “DNA damage response” or DDR. DNA itself is compacted into chromosomes whose tips are capped by specialized DNA-protein structures called telomeres. Using a custom-built super-resolution microscope for high-throughput imaging, EPFL scientists have now been able to investigate what happens to the sizes and shapes of human telomeres when aberrant telomere DDR is induced. The work is published in Genes & Development.
The telomeric DNA of human chromosomes is composed of a repeated sequence of nucleotides, which shortens in length during aging. Short telomeres trigger the DDR, which instructs the cells with short telomeres to stop dividing. This limits the growth of pre-cancerous lesions suppressing tumor formation. On the other hand, short telomeres also prevent the growth of healthy cells, thus limiting tissue renewal during aging. By removing just one of the shelterin proteins, scientists can confuse the cell into wrongly recognizing its own telomeres as DNA damage sites.
The labs of Joachim Lingner and Suliana Manley at EPFL used a super-resolution microscope that overcomes the limitations of light diffraction that prevent observation at scales below the wavelength of light. The microscopy technique they have employed is called “stochastic optical reconstruction microscopy” (STORM).
In STORM, fluorescent probes are attached to the sample — here, the telomeres of human chromosomes. The probes can switch between fluorescent and dark states while the microscope takes multiple snapshots, each one capturing only a sparse fraction of the fluorophores at a time. By analyzing these snapshots, researchers can determine the position of each fluorescent molecule with high precision. Finally, all the molecular positions are put together to reconstruct a final super-resolution image.
This approach allowed the scientists to study the shape and size of both functional and dysfunctional human telomeres that elicit DDR in the cell. They focused on a telomere protein complex called shelterin, which shelters the telomere itself by suppressing the effects of DDR on it. It was hypothesized that shelterin does this either by causing the telomere DNA sequence to form a protective “T-loop,” or by keeping the telomere from decompacting altogether.
By examining thousands of telomeres from many cells depleted of shelterin proteins, the scientists found the majority of telomeres that trigger DDR actually retained a normal size, meaning that DDR does not require decompaction of telomeres. Unfortunately, the diversity of telomere sizes and shapes meant that a large number of telomeres were required to draw any significant conclusions from the data — too many for the traditionally long acquisition times of STORM. To overcome this difficulty, the researchers utilized a new, large field-of-view STORM microscope recently developed at the EPFL to simultaneously image multiple cells at once and drastically increase the throughput of their measurements.
The findings refute a previous report in which damaged telomeres were proposed to expand up to 10-fold in size in order to make the telomeres accessible to the DDR machinery. The study suggests that telomeres are continuously monitored by the DDR machinery and that DDR activation does not involve telomere changes in size or shape. Instead, it seems that DDR is triggered by changes in structure and protein composition at the molecular level.
“The technical developments of the study set the stage to identify the mechanisms of telomere compaction in healthy cells and test its possible defects in disease,” says Joachim Lingner.
This study was funded by the Swiss National Science Foundation (SNSF), the NCCR RNA and disease network, the EC Seventh Framework Programme Programme (FP7), the Swiss Cancer League, EPFL, the ERC, and SystemsX.ch.
Reference
Aleksandra Vancevska, Kyle M. Douglass, Verena Pfeiffer, Suliana Manley, Joachim Lingner. The telomeric DNA damage response occurs in the absence of chromatin decompaction.Genes & Development 05 April 2017. DOI: 10.1101/gad.294082.116