Solvent-assisted Photoacidity in “Real-Time”
© 2013 EPFL
“Ultrafast solvent-assisted level crossing in 1-naphthol” scheduled for publication in Angewandte Chemie International edition (DOI: 10.1002/anie.201301931)
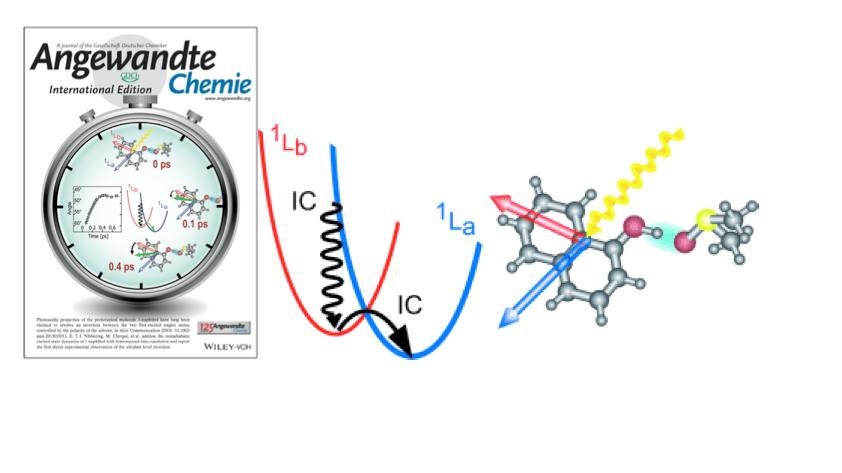
Upon absorption of light, molecules become excited to an electronic state. This excitation is often the trigger of a sequence of events where the molecular systems change their electronic excited states, en route to either the electronic ground state or to some photoproduct state. Such changes in electronic states are called non-adiabatic level crossing. Non-adiabatic electronic transitions govern the underlying dynamics in many photophysical and photochemical processes. Prime examples are the photoinduced electron and proton transfer, which lead to a rearrangement of charges. Ultrafast non-adiabatic electronic transitions are part of the protection mechanisms of DNA against UV radiation damage. Many such non-adiabatic electronic transitions are strongly influenced by the nature and motions of the surrounding solvent molecules.
In a collaborative effort, researchers from the Ecole Polytechnique Fédérale de Lausanne, the Max Born Institute for Nonlinear Optics and Short Pulse Spectroscopy (Berlin) and Yale University have - for the first time - determined the time scale for ultrafast electronic excited state level crossing in a classic case: the solvent-driven level crossing in 1-naphthol. This solvent driven level crossing, only observed in media of high polarity (water, alcohols), has been postulated as the underlying mechanism for the much higher photoacidity of 1-naphthol compared to other photoacid molecules.
Photoacidity is the phenomenon by which the acidity of an organic molecule is greatly enhanced after absorption of light. Based on estimates, the pKa-value of 1-napthol changes from 8-9 for the electronic ground state to about 3 for the electronic excited state. However, when dissolved in water, a pKa-value of 0.5 was found in the electronic excited state.
In a combined experimental and theoretical study, it has become clear that this apparent discrepancy is indeed caused by a solvent-controlled level crossing between the lowest two singlet excited electronic states. This level crossing is of ultrafast nature: in the polar solvent dimethylsulfoxide, as evidenced by the time-dependent fluorescence anisotropy, it occurs with a 60 fs time constant! Inspection of the transient behaviour of the IR-active C-O stretching mode shows that the hydrogen bond between 1-naphthol and dimethylsulfoxide molecules strongly increases, which is clearly not the case in weakly polar solvents, such as chloroform.
These findings will be of interest to any chemist, involved with experiments or theory of non-adiabatic electronic transitions. They offer a clear-cut example of solvent assisted non-adiabatic transitions, which are most probably the rule rather than the exception in liquid phase photochemistry. In addition, the results will propel many activities using state-of-the-art calculations of such dynamics. One aspect is that the electronic excited state structure calculations suggest a significant amount of excited state level mixing, controlled by the polarity of the surrounding solvent. A challenge will be to implement the dynamical nature of the solvent shells into a combined QM/MM description with which such an ultrafast nonadiabatic solvent-assisted level crossing can be described.