Shedding light on bacteria-killing viruses, one cell at a time
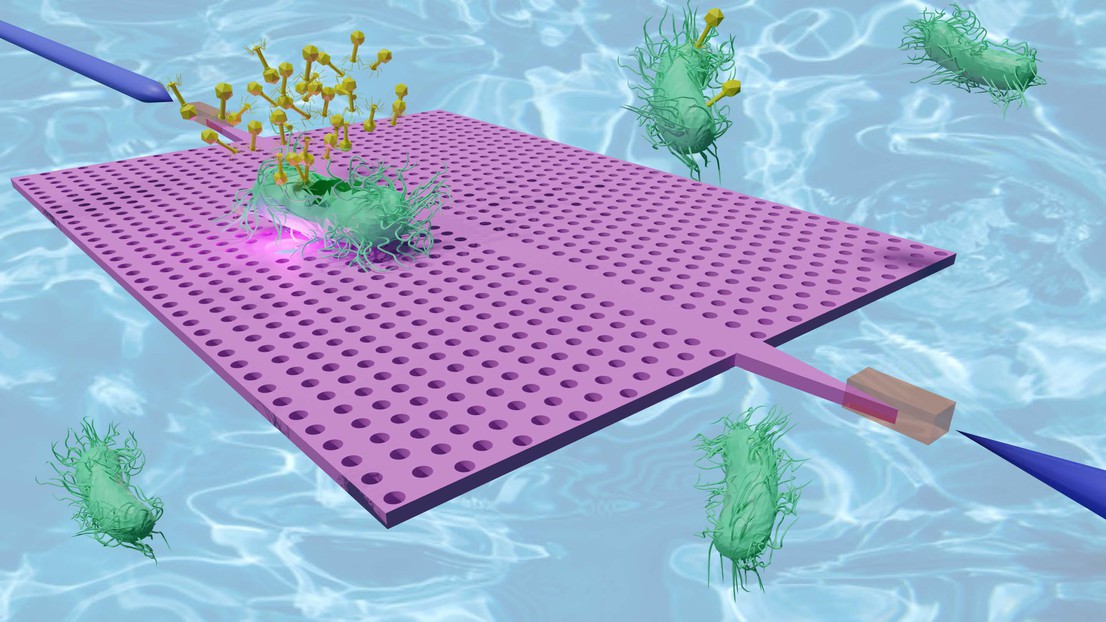
A burst bacterium on an H2 photonic crystal cavity (bright spot) releasing phages (yellow) in liquid. Credit: Enrico Tartari (EPFL).
EPFL researchers have developed a novel optical technique to study how bacteriophages—viruses that infect bacteria—attack single bacterial cells in real time. The breakthrough could improve phage therapy, a promising alternative to antibiotics in the fight against bacterial infections.
Bacteria and viruses have been at war for billions of years: Bacteria evolve defenses to survive viral attacks, while bacteriophages (phages) adapt to outmaneuver them. This microscopic battle rages everywhere, from the human gut to ocean water. But while phages have gained a lot of attention as a potential weapon against antibiotic-resistant bacteria, but monitoring their interaction in real-time with individual bacterial cells remained elusive.
Traditional methods of studying phage activity rely on bulk measurements that average the behavior of millions of bacteria. These methods, like turbidity assays and genetic sequencing, reveal whether phages are effective in killing bacteria but don’t capture the details of how they do it, especially at the single-cell level. Do all bacteria respond the same way? Can some resist better than others? And can we use these insights to refine phage therapy as a viable medical treatment?
Now, scientists at EPFL, in collaboration with the CEA Grenoble and the Lausanne University Hospital (CHUV) have developed an innovative approach to tackle this challenge: they built a tiny device that uses light to trap and study single bacteria that uses nanoscale light fields—trapped within photonic crystal (PhC) cavities—to hold and monitor single bacteria as they are attacked by phages. This technique allows researchers to track bacterial changes in real time, without the need for labels or chemical modifications.
The research was led by the group of Romuald Houdré at EPFL.
The device is based on photonic crystal (PhC) nanotweezers, which are tiny optical tools that use finely controlled light fields to trap tiny objects, such as bacteria, in place. The bacteria are then exposed to phages, and as the infection progresses, their response is continuously measured by analyzing the light intensity that holds them in place.
The setup distinguishes between different phases of infection—membrane degradation, swelling, and eventual lysis (bursting)—providing detailed insights into the destruction process.
The researchers used two types of PhC cavities, each with distinct optical properties: the L3 cavity allowed them to monitor changes in the bacterial outer membrane, while the H2 cavity could hold bacterial debris even after the cells had burst, offering a "post-mortem" look at the destruction process.
This technique let the scientists watch phages T1 and T4 attack Escherichia coli bacteria in real time—no labels or extra processing needed. They found that different phages break open bacteria in different ways. For example, T4-infected bacteria exploded more forcefully than T1-infected ones, which matched the fact that T4 produces more new viruses per infection. This means that not all phages work the same way, even when attacking the same type of bacteria, and that their effectiveness can vary even from one bacterial cell to another.
Phage therapy is an emerging alternative to antibiotics, especially for drug-resistant infections. However, the extreme specificity of phages—where one virus only infects a narrow range of bacteria—makes their medical use challenging. Doctors need to identify the right phage for each bacterial infection, which requires faster and more precise screening tools. The new optical method could help by allowing scientists to quickly test phage effectiveness at the single-cell level, paving the way for more personalized and efficient phage therapy approaches.
Swiss National Science Foundation (SNSF)
French RENATECH network
French National Research Agency (ANR, SUPPLY project)
Enrico Tartari, Nicolas Villa, Hugues de Villiers de la Noue, Simon Glicenstein, Emmanuel Picard, Pierre R. Marcoux, Marc Zelsmann, Emmanuel Hadji, Grégory Resch, and Romuald Houdré. Monitoring of Single-Cell Bacterial Lysis by Phages within Integrated Optical Traps. Advanced Optical Materials 25 January 2025. DOI: 10.1002/adom.202402586