Self-assembly at Hydrophobic Interfaces Explained by Machine Learning
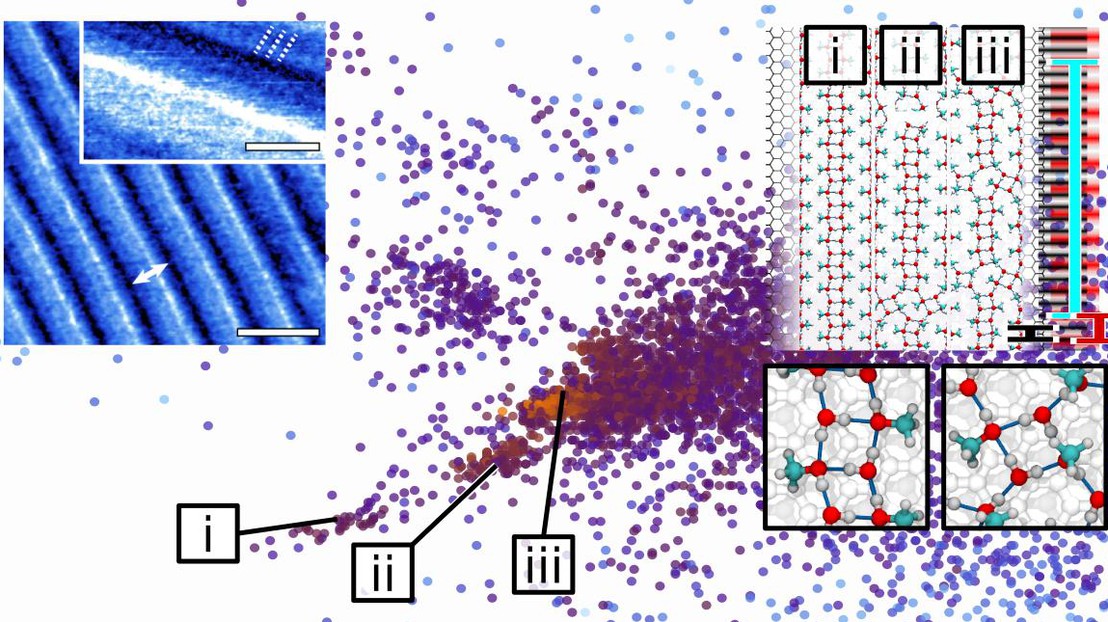
A map of MeOH/Water self-assembly © M.Ceriotti / EPFL 2016
Experiments at SUNMIL/EPFL and at Durham University demonstrate the formation of nanoscopic structures at the interface between graphite and a mixture of water and methanol. Atomistic simulations at COSMO/EPFL, in conjunction with a machine-learning analysis of the conformational landscape, demonstrate that these nanostructures are arrays of defects that arise because of the mismatch between the H-bond network of the solution and the interaction with the substrate.
Atomic-force microscopy imaging of the interface between a MeOH/water solution and graphite revealed the thermally-activated nucleation of ordered domains, containing regular stripes with a period of about 10nm. This observation is remarkable, because this nanoscopic length scale is much larger than either the periodicity of the substrate or the size of the molecules involved.
An explanation of the origin of this self-assembled nanostructured was obtained thanks to a combination of multi-scale atomistic simulations and a machine-learning analysis of the simulation outcome. Water and methanol - that are known to form a highly frustrated, inhomogeneous H-bond network, show a tendency to generate 1D structures at a hydrophobic interface. At the same time, the interaction with graphite means that methanol molecules tend to arrange themselves with a periodicity that matches that of a graphene sheet. The small mismatch between the characteristic scale of the H-bond network, and that of graphite, lead to the formation of periodic arrays of defects, on a much larger length scale that the size of the molecular components. The recognition of these defects patterns was made possible by the use of advanced dimensionality-reduction algorithms, that sorted through the hundreds of thousands of locally stable configurations that are found by simulating this extremely glassy interfacial system.
The explanation of the origin of these self-assembled nanostructures could have implications for the behavior of H-bonded solutions at hydrophobic interfaces, and in particular for fuel cells applications. An article describing these experimental findings, accompanied by the theoretical analysis, have been recently published on Nature Communications.