Scientists unveil a DIY structured-illumination microscope

© 2023 LBMI
Scientists at EPFL have published a guide to building an add-on that turns a standard optical microscope into an instrument capable of producing super resolution, 3D images of cells, organoids, and embryos.
For hundreds of years, the optical microscope was the only tool available to scientists wanting to study the movement of cells, bacteria and yeast. But the diffraction of light made it impossible to observe objects at resolutions of less than 100 nm because the resulting images were too blurry to be of any use. This physical limit – known as the diffraction barrier – was finally overcome around 15 years ago with the development of super-resolution microscopy, allowing scientists to peer deep inside living specimens, study the behavior of organelles, and observe how cells interact with viruses, proteins and drug molecules. One of these new methods, known as structured illumination microscopy (SIM), is highly prized by researchers because it produces high-resolution and high-contrast images with low photon exposure. Despite the advent of nanometer-resolution electron microscopes, optical imaging continues to play a key role in life-science research: it offers greater flexibility in terms of equipment and lets scientists observe live samples in normal developmental conditions. However, cost and availability constraints mean that SIM imaging remains out of reach for many. To get around this problem, scientists at EPFL’s Laboratory for Bio- and Nano-Instrumentation (LBNI) within the Interfaculty Institute for Bioengineering (IBI) at EPFL’s School of Engineering (STI), have developed a way to transform a standard optical microscope into a high-resolution device using inexpensive, commercially available components. The team has published a detailed how-to guide in open-access format, along with a series of video tutorials.
A compact microscope that non-experts can build and use
SIM overcomes the diffraction barrier by reconstructing the areas of high spatial frequencies that normally appear blurred when viewed through a conventional optical microscope. This method offers a twofold increase in resolution, enabling scientists to observe details as small as 100 nm across. SIM works by projecting a standard illumination pattern, such as a grid, onto a sample. Images, captured with different illumination patterns, are then processed by an algorithm to produce a higher-resolution reconstruction, harnessing the moiré effect.
Back in 2019, PhD student Mélanie Hannebelle needed a microscope with precisely this capability for her research. That’s when she came up with the idea of building one herself for the LBNI. Other labs had already made similar devices, but they were complex, cumbersome, and difficult to reproduce. Hannebelle wanted to design a more compact alternative that non-experts could build and use and that didn’t require expensive upkeep and maintenance. “We sourced electronic components of the kind used to make the video projectors you see in classrooms,” says Georg Fantner, a professor at LBNI. “We altered and arranged them, so they were capable of projecting a light pattern onto a sample.”
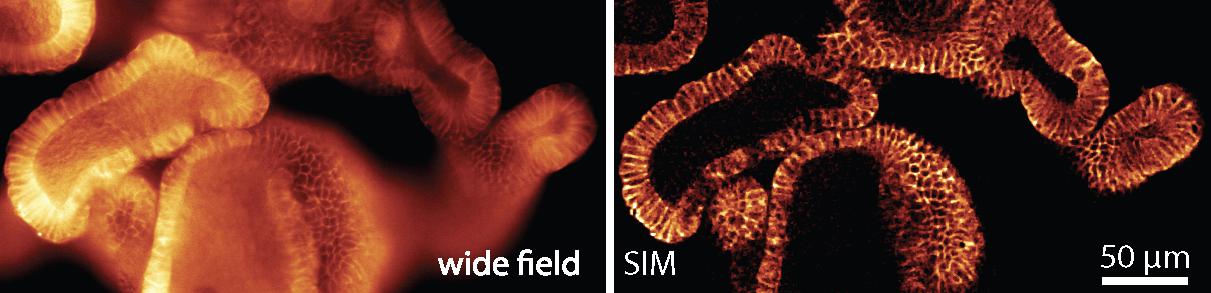
Tested and approved by life-sciences researchers
The LBNI team wanted to find out whether their new microscope was a viable and practicable alternative. So they asked other labs to test it. They teamed up with the groups of Prof. Andrew Oates, Prof. Matthias Lutolf, Prof. John McKinney and Prof. Aleksandra Radenovic to test the instrument on real world research samples. “Our colleagues asked us questions, told us about their needs, and shared their samples with us,” says Prof. Fantner. “We were eager to find out whether and how our instrument could help them in their research.” The feedback was overwhelmingly positive, and the team secured an EPFL Open Science grant so they could share their instrument in open-hardware format. Turning the device into something other labs could reproduce, with instructions detailed enough that colleagues wouldn’t give up part-way through the process, proved to be a painstaking and time-consuming process. Esther Raeth, another PhD student in the lab, put together detailed instructions, equipment lists, and video tutorials for publication online. “The only prerequisite for our system is a high-quality optical microscope – something most labs already have,” explains Prof. Fantner.
The OpenSIM does not aim to compete with more sophisticated instruments. For instance, the approach has a lower modulation contrast than commercially available equivalents, which constrains the resolution-gain to a factor of 1.7x compared to the theoretical 2x. But it serves its intended purpose: to make SIM technology available to labs that need it only occasionally or that simply can’t afford to spend CHF 500,000 or more on a commercial-grade model. The LBNI team is pressing ahead with efforts to bring its work to a wider group of scientists and build a community of users to share their experiences. “Since the paper was shared on BioRxiv.org, I’ve been contacted by several people who are interested in the idea and want to know more about how to build their own OpenSIM.” says Prof. Fantner.
EPFL’s Open Science grants support projects that create innovative tools, build essential infrastructure and cultivate a community that embraces the FAIR principles (Findable, Accessible, Interoperable and Reusable). Learn more at: https://www.epfl.ch/research/open-science/os-grants/
Mélanie T. M. Hannebelle, Esther Raeth, Samuel M. Leitao, Tomáš Lukes, Jakub Pospíšil, Chiara Toniolo, Olivier F. Venzin, Antonius Chrisnandy, Prabhu P. Swain, Nathan Ronceray, Matthias P. Lütolf, Andrew C. Oates, Guy M. Hagen, Theo Lasser, Aleksandra Radenovic, John D. McKinney & Georg E. Fantner. Open-source microscope add-on for structured illumination microscopy. Nature communications 26 January 2024. DOI: https://doi.org/10.1038/s41467-024-45567-7