Rethinking bacterial persistance
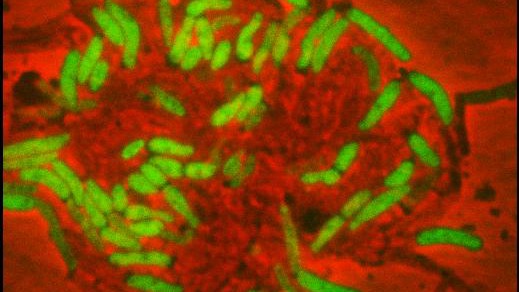
Bacterial ife, growth and death is now to be seen more accurately. © 2012 LMIC / EPFL
EPFL scientists used microfluidics to observe the behavior of individual tuberculosis-like bacteria in the presence of antibiotics. Their observations call into question the prevailing theory of bacterial resistance, and they have proposed a new explanation for why some bacteria become resistant. The research is published January 4, 2013 in the journal Science.
It’s often difficult to completely eliminate a bacterial infection with antibiotics; part of the population usually manages to survive. We’ve known about this phenomenon for quite some time, dating back nearly to the discovery of penicillin. For more than 50 years, scientists have believed that the resistant bacteria were individuals that had stopped growing and dividing.
Up to now, in fact, it hasn’t been possible to track the growth of cells before and after their exposure to antibiotics, which makes any analysis of the phenomenon quite imprecise. “Using microfluidics, we can now observe every bacterium individually, instead of having to count a population,” says John McKinney, director of EPFL’s Microbiology and Microsystems Laboratory (LMIC).
Active survivors
This new tool has revealed quite a few surprises. “We thought that surviving bacteria made up a fixed population that stopped dividing, but instead we found that some of them continued to divide and others died. The persistent population is thus very dynamic, and the cells that constitute it are constantly changing – even though the total number of cells remains the same. Because they’re dividing, the bacteria can mutate and thus develop resistance in the presence of the antibiotic,” explains LMIC scientist Neeraj Dhar.
This point is extremely important. “We were able to eliminate a purely genetic explanation of the phenomenon,” continues Dhar. In other words, “a population of genetically identical bacteria consists of individuals with widely varying behavior. Some of them can adapt to stressors that they have not previously encountered, thanks to the selection of persistent individuals. This could lead to a revision of the entire theory of adaptation,” says McKinney.
Intermittent efficiency
The EPFL scientists were particularly interested in a relative of the tuberculosis bacterium. Their observations enabled them to formally challenge the argument that persistent bacteria are those that have stopped growing and dividing. “We were able to reveal the role of an enzyme whose presence is necessary in order for the antibiotic to work, and show that the bacilli produced this enzyme in a pulsatile and random manner,” explains Dhar. “Our measurements showed that bacterial death correlated more closely with the expression of this enzyme than with their growth factor.” The research is being published this week in Science magazine.
These conclusions could mark the beginning of a new theory of bacterial resistance, or perhaps even introduce a new view of how such resistance evolves. Further research is being done using other microorganisms, such as tuberculosis and E. coli bacteria. The persistence of some cancer cells to treatment could also be studied in a different manner. “It’s a new approach for trying to figure out why some infections are so difficult to eliminate. The techniques we’ve developed for this study are now also being used to develop new antibiotics, in collaboration with pharmaceutical companies,” says McKinney, adding that “it is the microengineering expertise at EPFL that has enabled us to create these innovative tools and open up new avenues for investigation.”