Reading our brain chemistry
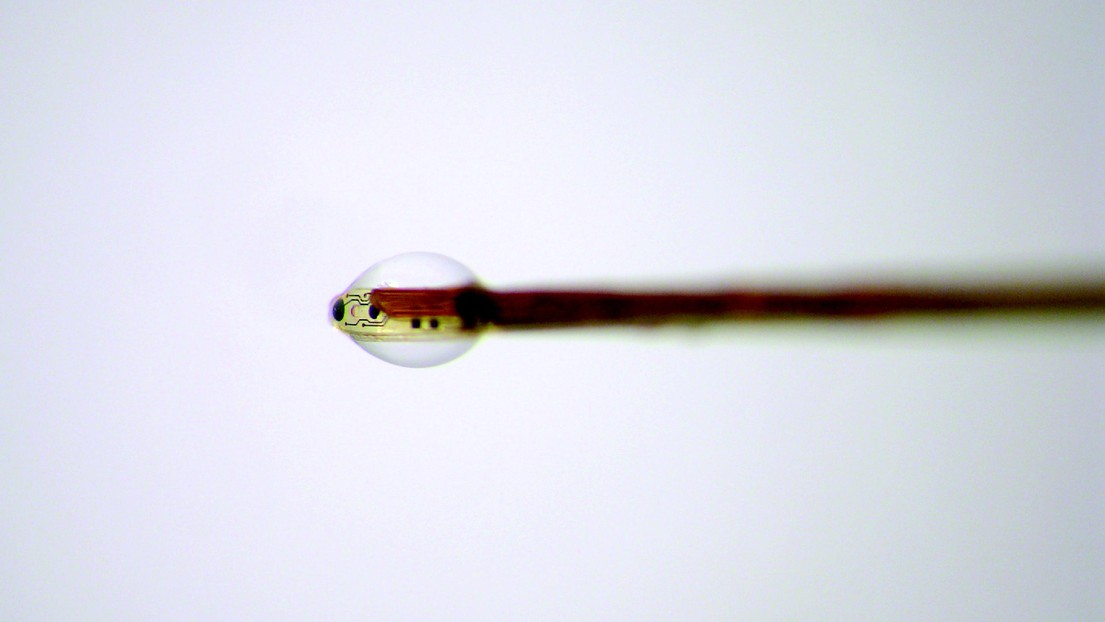
© Guillaume Petit-Pierre - Perfusion microdroplet allowing the extraction of interstitial liquid using the system developed by EPFL researchers.
Researchers at EPFL have developed a new device and analysis method that let doctors measure the neurochemicals in a patient’s brain. The Microsystems Laboratory 4 (LMIS4)'s system involves collecting microdroplets of cerebral fluid and analyzing them to obtain chemical data that can help doctors diagnose and treat neurodegenerative diseases.
Neurologists often use electrical impulses to stimulate and read brain signals. But the chemicals that neurons produce in response to these impulses are poorly understand at this point, even though they can provide valuable information for understanding the mechanisms behind neurodegenerative diseases like Alzheimer’s and Parkinson’s. “Neurons can be read two ways: electrically or chemically,” says Guillaume Petit-Pierre, a post-doc researcher at LMIS4 and one of the study’s authors. “Reading their electrical behavior can provide some limited information, such as the frequency and pace at which neurons communicate. However, reading their neurochemistry gives insight into the proteins, ions and neurotransmitters in a patient’s cerebral fluid.” By analyzing this fluid, doctors can obtain additional information – beyond that provided by neurons – and get a complete picture of a patient’s brain tissue metabolism.
Collecting information through microchannels
The EPFL researchers developed a system that can both collect a patient’s neurochemical feedback and form electrical connections with brain tissue. Their device is made up of electrodes and microchannels that are about half a hair in diameter. Once the device is placed inside brain tissue, the microchannels draw in cerebral fluid while the electrodes, which are located right at the fluid-collection interface, make sure that the measurements are taken at very precise locations. The microchannels subsequently create highly concentrated microdroplets of cerebral fluid. “The microdroplets form directly at the tip of the device, giving us a very high temporal resolution, which is essential if we want to accurately analyze the data,” says Petit-Pierre. The microdroplets are then placed on an analytical instrument that was also developed by scientists at the LMIS4 and the nearby University Centre of Legal Medicine which has expertise in this type of complex analysis. As a last step, the microdroplets are vaporized with a laser and the gas residue is analyzed. Both the researchers’ device and their analysis method are totally new. “Today there is only one method for performing neurochemical analyses: microdialysis. But it isn’t very effective in terms of either speed or resolution,” says Petit-Pierre. Another advantage of the researchers’ method is that it is a minimally invasive way to collect data. Currently scientists have to work directly on the brains of rats afflicted with neurodegenerative diseases, meaning the rats must be sacrificed to take the measurements. Their research was published in Nature Communications.
Direct applications
For this study, the researchers focused primarily on the calcium, sodium, potassium and other ions in cerebral fluid. They worked with EPFL’s Neurodegenerative Disease Laboratory to compare the measurements they took on rats with those reported in the literature – and found that the results were well correlated. The next step will be to develop a method for analyzing the proteins and neurotransmitters in cerebral fluid, so that their implications in neurodegenerative diseases can be further studied. “Doctors could measure neurochemical responses to help them make diagnoses, such as for epilepsy, when they use electricity to measure signals from a patient’s cortex,” says Guillaume, “or to improve the efficiency of treatments like deep brain stimulation (DBS) for Parkinson’s disease.” Their research could also soon find direct applications in other medical fields. Guillaume currently works on a start-up project to develop a catheter for patients affected by hemorrhagic stroke. Based on a similar technology, his catheter would let doctors treat a common yet serious complication of this condition and thereby reduce the risk of death.
This research was carried out jointly by EPFL’s Laboratory of Microsystems 4 (LMIS4), EPFL’s Neurodegenerative Disease Laboratory (LEN), the Unit of Toxicology at the University Centre of Legal Medicine (CURML, CHUV and HUG) and the University of Lausanne’s Faculty of Biology and Medicine (FBM, UNIL).
“In vivo neurochemical measurements in cerebral tissues using a droplet-based monitoring system”Guillaume Petit-Pierre, Philippe Colin, Estelle Laurer, Julien Déglon, Arnaud Bertsch, Aurélien Thomas, Bernard L. Schneider and Philippe Renaud

