Radicals from sunlight transform sulfonamide antibiotics in nature
© 2013 EPFL - LMCE
EPFL researchers shed light on the degradation of sulfonamide antibiotics in aquatic environments.
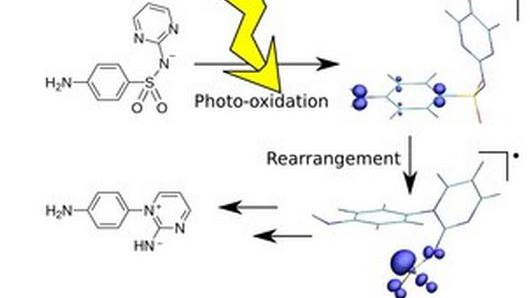
When sulfonamide antibiotics break down in lakes and rivers, they can do so along several alternative degradation pathways. One of them leads to an often-observed degradation product, which loses a sulfur dioxide group and is rearranged compared to its original configuration. Until now, reports on how this degradation compound could be formed offered differing hypothesis.
In collaboration with the experimentalist group of Kristopher McNeill at ETH Zurich, Tentscher and Arey used quantum chemical computer modeling and laser photolysis techniques to establish a detailed reaction mechanism that leads up to this observed degradation product. Its first step involves a reaction with free radicals, reactive chemical species that are present almost everywhere in the sunlit environment. “We were particularly interested in studying this reaction in the context of aquatic micropollutants, but the same reaction could occur in the body, in principle,” says Arey.
As the authors write, a better understanding of the reactions at play – and the means to simulate them using computer models – will help in the development of future sulfonamide antibiotics.