Proteins à la carte expand beyond Nature's repertoire
© 2020 EPFL
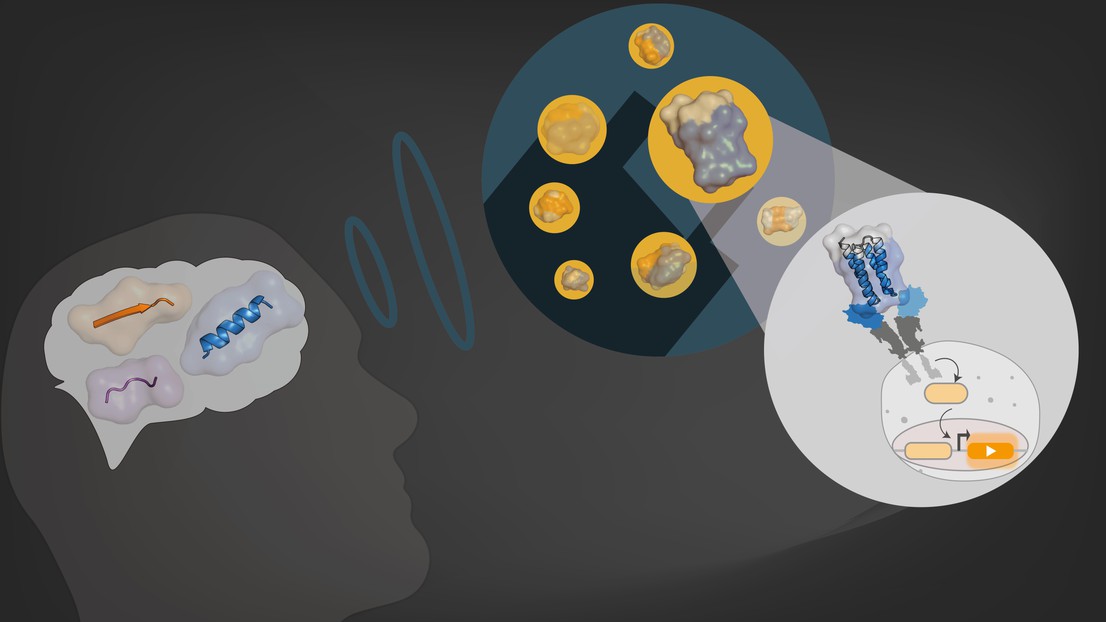
School of Engineering’s scientists have developed a new computational method to design artificial proteins with functions that are not found in nature.
Naturally evolved proteins are the nuts and bolts of life. However these proteins were shapped by evolution for very specific tasks related to the survival of their host organisms. Beyond, naturally evolved proteins there is an extensive universe of proteins of which we can only imagine their potential applications.
“Imagine building a car by putting together Lego bricks. That’s basically what we do at the Laboratory of Protein Design & Immunoengineering, except with proteins,” says Fabian Sesterhenn, a former PhD student in the lab. He’s part of a research team, headed by Professor Bruno Correia at the School of Engineering, that has already used computational design to engineer proteins for a vaccine against the respiratory syncytial virus (RSV). Today they are once again creating novel functional proteins with their computers, but this time for different applications. Their research has just been published in Nature Chemical Biology.
See also: Designing vaccines from artificial proteins
Most scientists currently use natural proteins and re-design them for other applications. “They basically repurpose nature’s proteins,” says Che Yang, also former PhD student in the lab. But he and his colleagues have developed a bottom-up approach. Instead of working with natural proteins, they assemble their building blocks using a computer program, which can simulate up to many thousands of possible combinations. “We build and test many of the possible combinations in our lab and select a few that meet all the criteria we’re looking for,” he explains.
Sensing anti-viral antibodies
The scientists’ new method is effective for creating novel functional proteins. But their synthesized proteins – unlike natural ones – can also serve as biosensors. “One application that we used these proteins for is as a biosensor to detect particular anti-viral antibodies in the bloodstream,” explains Che Yang.
A joint research effort
Further, the de novo proteins, designed by Yang and colleagues, can also control the signaling of receptors located on a cell’s surface. “Receptors are used to provide cells with important information about their surroundings. With our proteins, we can activate or deactivate specific receptors,” says Yang. Both these applications were developed in collaboration with the laboratories headed by Martin Fussenegger at ETH Zurich and Maarten Merkx at Eindhoven University of Technology in the Netherlands.
Over billions of years of evolution, nature has created many millions of proteins essential for the function of living systems. “And now we are one step closer to resemble the natural procees by using protein design to craft our own functional proteins. In the near future, we may likely create many other proteins that can have an impact in our daily lives,” concludes Correia.
The computational simulations were facilitated by the CSCS Swiss National Supercomputing Centre as well by SCITAS at EPFL. This work was supported by the Swiss initiative for systems biology (SystemsX.ch), the European Research Council (Starting grant - 716058), the Swiss National Science Foundation (310030_163139), the NCCR Molecular Systems Engineering and the NCCR Chemical Biology. F.S. was supported by an SNF/Innosuisse BRIDGE Proof-of-Concept grant, and J.B. was funded by the EPFL Fellows postdoctoral fellowship. T.K. received funding from the Cluster of Excellence RESIST (EXC 2155) of the German Research foundation and from the German Center of Infection Research, J.T.C. was supported by the ERA-Net PrionImmunity project 01GM1503 of the German Federal Ministry of Education and Research.