Propeptide driven folding of proaerolysin.
© 2011 EPFL
Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin.
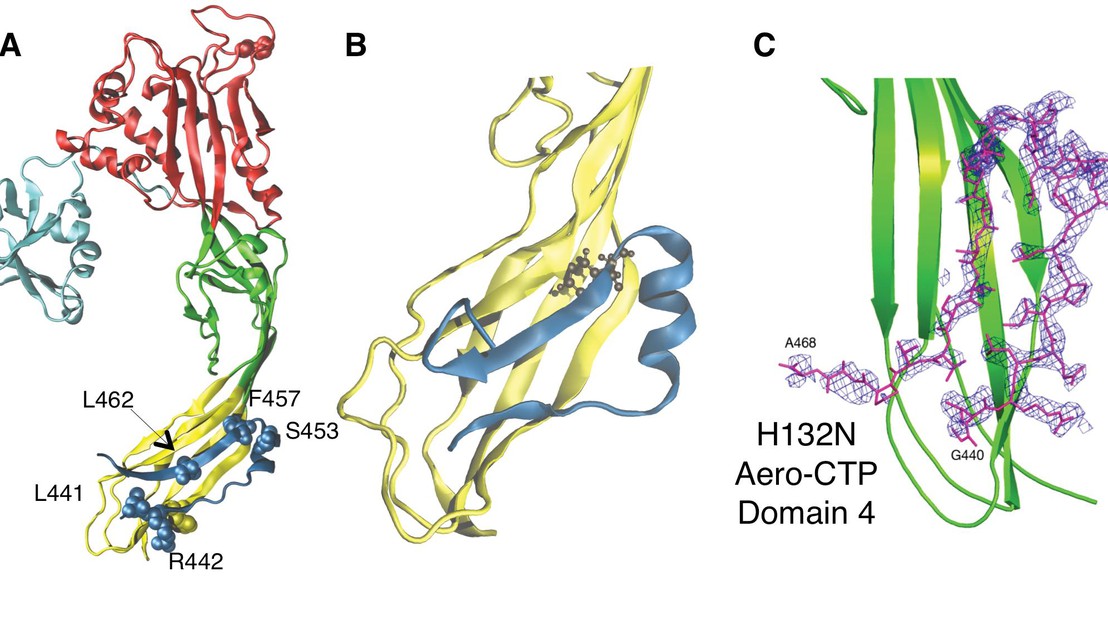
Throughout evolution, one of the most ancient forms of aggression between cells or organisms has been the production of proteins or peptides affecting the permeability of the target cell membrane. This class of virulence factors includes the largest family of bacterial toxins, the pore-forming toxins (PFTs). PFTs are bistable structures that can exist in a soluble and a transmembrane state. It is unclear what drives biosynthetic folding towards the soluble state, a requirement that is essential to protect the PFT-producing cell. The group of Prof. Gisou van der Goot (van der Goot Lab) has investigated the folding of aerolysin, produced by the human pathogen Aeromonas hydrophila, and more specifically the role of the C-terminal propeptide (CTP). They show that the CTP is crucial for the control of the aerolysin activity, since it protects individual subunits from aggregation within the bacterium and later controls assembly of the quaternary pore-forming complex at the surface of the target host cell. The CTP is the first example of a C-terminal chain-linked chaperone with dual function.
Ioan Iacovache er al, PLoS Pathogens, doi:10.1371/journal.ppat.1002135 (2011)