Photoelectrochemical CO2 Reduction Directly on CIGS Surface
© 2023 The authors/EPFL
LIMNO researchers have demonstrated that photoelectrochemical CO2 reduction occurs with high selectivity at a direct CuInGaS2/electrolyte junction when the electrolyte conditions are correctly tuned.
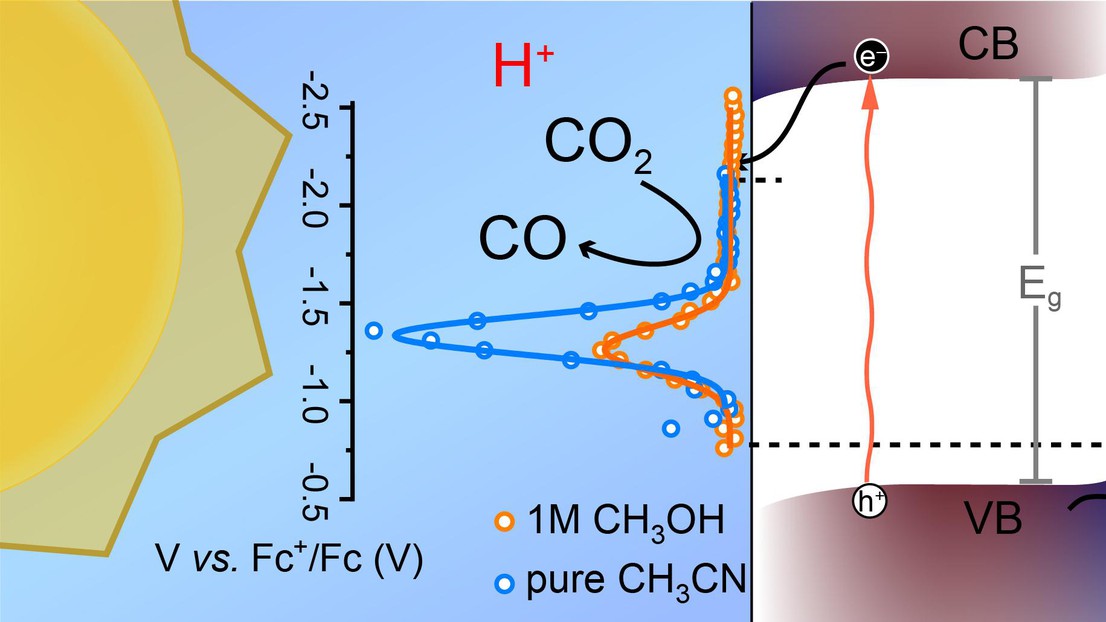
Photoelectrochemical (PEC) CO2 reduction has received considerable attention given the inherent sustainability and simplicity of directly converting solar energy into carbon-based chemical fuels. However, complex photocathode architectures with protecting layers and cocatalysts are typically needed for selective and stable operation. In a new report from the LIMNO lab at EPFL, researchers show that bare CuIn0.3Ga0.7S2 photocathodes can drive the PEC CO2 reduction with a benchmarking 1 Sun photocurrent density of over 2 mA/cm2 (at −2 V vs Fc+/Fc) and a product selectivity of up to 87% for CO (CO/all products) production while also displaying long-term stability for syngas production (over 44 h). Importantly, spectroelectrochemical analysis using PEC impedance spectroscopy (PEIS) and intensity-modulated photocurrent spectroscopy (IMPS) complements PEC data to reveal that tailoring the proton donor ability of the electrolyte is crucial for enhancing the performance, selectivity, and durability of the photocathode. When a moderate amount of protons is present, the density of photogenerated charges accumulated at the interface drops significantly, suggesting a faster charge transfer process. However, with a high concentration of proton donors, the H2 evolution reaction is preferred. Overall, these findings support the feasibility of direct semiconductor/electrolyte junctions to drive PEC CO2 reduction, although further studies are needed to suppress the surface recombination sites and the competing HER reaction.
This work was supported by the Gaznat-EPFL research program. The Swiss National Science Foundation (SNSF) also partially supported the work under the Ambizione Energy grant (PZENP2_166871).
ACS Energy Lett. 2023, 8, 1645–1651