OST & EPFL increase efficiency for renewable energy conversion to 70%
© 2023 OST & EPFL
Like all major changes, the energy transition is complicated in detail. For example, renewable energy sources such as solar and wind already produce significantly cheaper electricity than nuclear power plants. So far, however, it is expensive to store this energy. Research teams around the world are therefore working on concepts for storing renewable energy as efficiently and cheaply as possible. Working together, two research teams at the Ostschweizer Fachhochschule (OST) and at the School of Engineering’s Group of Energy Materials (GEM) at EPFL Sion have now managed to increase the efficiency of a central process in the storage of renewable energy as synthetic fuels from 50% to 70%. An economically favorable, long-term storage of renewable energy is thus within reach.
A purely electrical energy future is unlikely. While cars or building heating can more easily shift from fossil-based energy sources to electricity, other sectors remain dependent on fuels. Industrial processes such as concrete production, air and heavy-duty traffic, and shipping require significantly higher energy density than that afforded by rechargeable batteries. In other words, the goal is to obtain as much energy as possible from as little energy carrier mass as possible (see box below).
Against the backdrop of the global goal of reducing CO2 emissions to zero, the only solution to this problem so far is the storage of renewable energy in the form of synthetic fuels. This is because when producing diesel from renewable electricity, for example, just as much CO2 is removed from the atmosphere as is released again during combustion.
Goal achieved: High efficiency for industrial production
The raw material for all synthetically produced chemical energy carriers (so-called e-fuels) is hydrogen. For this to be produced sustainably, water is usually split into hydrogen and oxygen by means of electrolysis using regenerative electricity. If the hydrogen is then combined with CO2, for example from waste incineration plants, various hydrocarbons like gaseous methane or liquid methanol can be synthesized. Methane can be used in the same way as natural gas, and methanol is the base material for many liquid hydrocarbons – from petrol or diesel to kerosene and basic materials for the chemical industry.
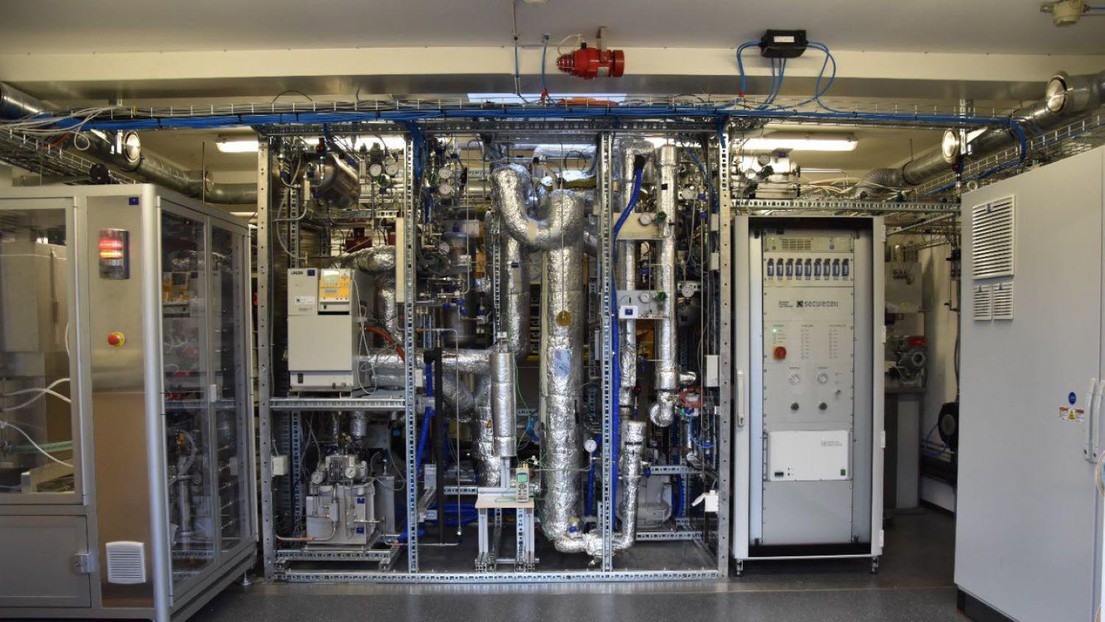
A research team from OST’s Institute for Energy Technology (IET) and EPFL-GEM has been working on high-temperature electrolysis since 2017 to make the conversion of electricity into methane more efficient. The target was to increase the overall conversion efficiency from the current 50% to 70% for an industrial-scale plant.
"This means that 70% of the electricity invested can be stored in the methane," explains IET project manager Luca Schmidlin.
This significant increase was measured in the IET's Power-to-X research facility in Rapperswil-Jona. The prototype of the high-temperature electrolysis, supplied by EPFL-GEM and based on technology from SolydEra (Yverdon), was compared on a demonstration scale (around 15 kW) with an industrial PEM electrolysis in combination with that of catalytic methanation. The main difference between the two electrolysis technologies is that high-temperature electrolysis uses highly heated water vapor as the feedstock, while PEM electrolysis uses liquid water.
Save energy and make it economical
The core of the innovative demonstration plant is that the energy required for steam production comes from the reaction heat generated by the downstream methane synthesis. The resulting heat is thus used instead of escaping. In tests in the partial load range, around 3/4 of the amount of steam required for efficient high-temperature electrolysis could be produced. Additional tests for optimized heat integration at EPFL show that it is possible to generate 100% of the required steam thanks to the heat from methane synthesis. The operation could be maintained for several hours without any problems. First comparisons between the two operating modes show that thanks to the new technology, operation was a whopping 25 percentage points more efficient.
The project team is therefore very close to the targeted goal of increasing the overall efficiency of power-to-gas conversion (electricity storage in the form of methane) from the current 50% to 70%. The tests will be repeated in the first half of 2023 with a freshly revised system to confirm the data from the first two test series. If the results can be confirmed, this would represent an important step for the storage of renewable energy.
"Thanks to the design of our research platform as an industry-oriented demonstration system, the results can be transferred 1:1 to large-scale industrial systems," says Schmidlin.
Increasing the efficiency of renewable electricity conversion into renewable energy sources is an economically relevant factor when it comes to storing surplus renewable energy in the summer for use in winter. With the success of the IET and EPFL teams in increasing efficiency of the production of climate-neutral, synthetic methane, it will be possible, for example, to produce synthetic natural gas on an industrial scale. Natural gas consists mainly of methane.
BOX : Energy density and battery production challenges
A kilogram of diesel contains about 40-50 times as much energy as a kilogram of a modern lithium-ion battery. For example, an electric truck would have to use a 4,000 to 5,000 kg battery instead of a 100 kg diesel tank to carry the same amount of energy. Even though electric vehicles are more efficient than combustion engines, this ratio can only be compensated for by reducing the range or the possible transport capacity – which in turn reduces possible uses and economies, leading to higher costs.
In addition, excess electrical energy from renewable sources can be stored in the summer over longer periods of time more cheaply and flexibly in the form of synthetic fuels than batteries. Unlike electricity, fuels do not lose any energy during storage and transport over long distances. Depending on the type of fuel, they can also be converted back into electricity in (gas) power plants, used to refuel vehicles, or distributed throughout Switzerland via the gas network.
Finally, synthetic fuels can be used in existing infrastructure (e.g., in the gas network, in vehicles, or in industrial processes) without any conversion investments, expensive industrial battery system production, or portable batteries.