Organic spacers create free charges in layered halide Perovskites
© 2024 EPFL
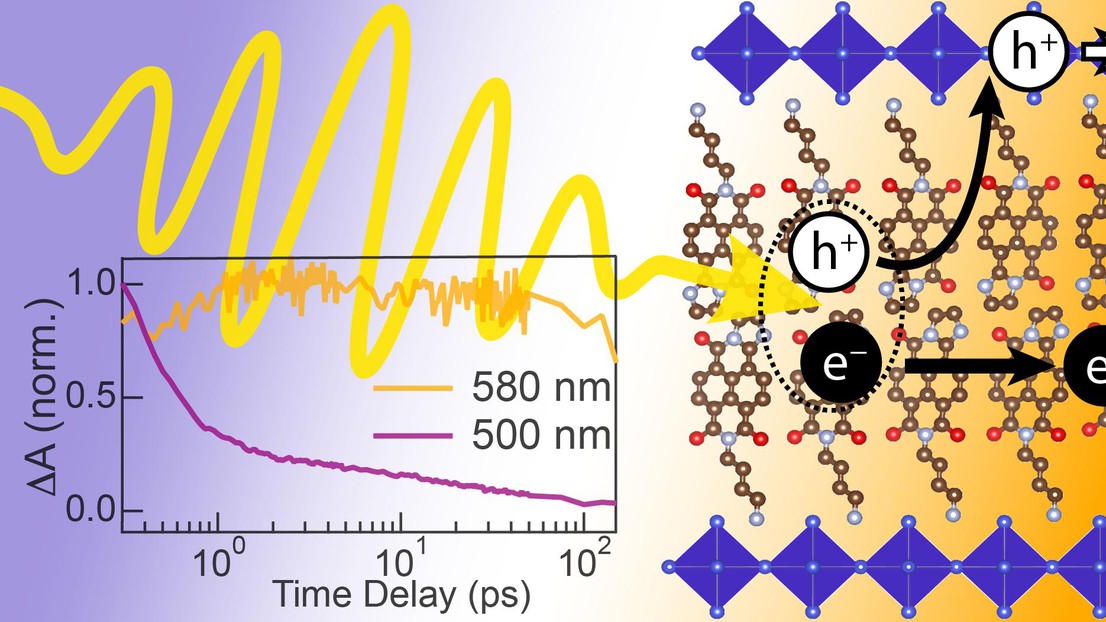
The LIMNO lab introduces a new lead-halide-based Ruddlesden–Popper perovskite structure based on a visible-light-absorbing naphthalene-iminoimide cation. The optoelectronic properties of this new material represent an important step toward enhancing light harvesting and affording the spatial separation of charge carrier transport in stable layered perovskite-based devices.
Incorporating organic semiconductor building blocks as spacer cations into layered hybrid perovskites provides an opportunity to develop new materials with novel optoelectronic properties, including nanoheterojunctions that afford spatial separation of electron and hole transport. However, identifying organics with suitable structure and electronic energy levels to selectively absorb visible light has been a challenge in the field. In a new paper published in JACS, the LIMNO lab, together with colleagues from Photochemical Dynamics Group, Helmholtz Zentrum Berlin für Materialien und Energie, and the University of Bern introduce a new lead-halide-based Ruddlesden–Popper perovskite structure based on a visible-light-absorbing naphthalene-iminoimide cation (NDI-DAE). Thin films of (NDI-DAE)2PbI4 show a quenched photoluminescence and transient absorption dynamics consistent with the formation of a charge transfer state or free charge carriers when either the inorganic or organic layer is photoexcited, suggesting the formation of a type II nanoheterostructure. Time-resolved microwave conductivity analysis supports free charge generation with sum mobilities up to 4 × 10–4 cm2 V–1 s–1. Mixed halide (NDI-DAE)2Pb(IxBr1–x)4 films show modified inorganic layer band gaps and a photoluminescent reversed type I nanoheterostructure with high bromide content (e.g., for x = 0). At x = 0.5, transient absorption and microwave conductivity measurements provide strong evidence that selective visible-light absorbance by the NDI-DAE cation generates separated free carriers via hole transfer to the inorganic layer (leaving photogenerated electrons in the organic layer).