New technology gives implants a protective covering
© 2012 labseed
A technology developed by labseed, an EPFL spin-off, could prevent most breast implant rejections. The final product should be commercially available as early as 2013.
More than a quarter of all breast implants must be removed within four years, because neighboring tissues develop a rigid envelope of fibrous tissue to protect themselves from the foreign body. Labseed, a start-up company based in the Science Park in Ecublens, has developed a protective covering made up of a nanostructured surface and a layer of collagen that will prevent the body from rejecting the implant.
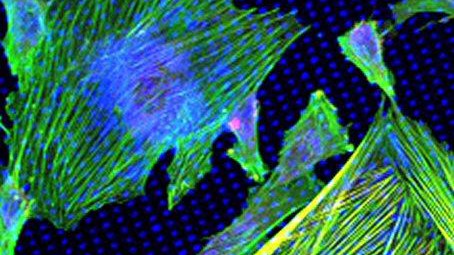
Our bodies treat all medical or plastic surgery devices- things like breast implants, knee and hip replacements, pacemakers and insulin pumps - as foreign invaders. We’re equipped with a complex surveillance system for recognizing and then eliminating them. In the empty intracellular space between the device and neighboring tissues, special cells that are in charge of this reaction, called fibroblasts, assemble to deal with the intruder. In certain cases, sometimes even several years after the implant is placed, they surround it and cover it in a very hard capsule. In addition to its unattractive appearance, particularly in breast implants, this reaction can also prevent the implant from functioning properly, such that in a quarter of patients, implants must be removed within four years after implantation.
Labseed co-founders Hicham Majd and Giorgio Pietramaggiori have developed a technique that eliminates this problem, and in vivo results have demonstrated the effectiveness of their new approach. The major advance of their technique is that it renders foreign bodies virtually “invisible” to cells that are watching out for invaders. This new surface treatment technology, called “MYcoat,” was developed from research done in EPFL’s Laboratory of Cell Biophysics and improved in the Laboratory of of Biomechanical Orthopedics in the frame of a CTI discovery project. It combines nano/microtechnology and biochemistry.
Mycoat structures the surface of a medical device or implant at a nanometer-level precision. The implant is then coated with collagen. In this way, neighboring cells are no longer in direct contact with the foreign body but with the nanometer-structured, collagen-coated surface. To the cells, this protective coating looks like just a new extracellular matrix, which they see as normal tissue. The fibroblasts will thus adhere quite naturally to the object, as if it was an integral part of the patient’s body.
The procedure has been finalized for silicon implants, but is also applicable for titanium elements. “Collagen, which makes up 80% of our bodies, is particularly well tolerated,” says Pietramaggiori.
This technique improves the interaction between the medical implant and the human body. Its simplicity of application makes it particularly attractive for major medical prosthesis and implant manufacturers. “Discussions are underway with major breast implant manufacturers,” the co-founder adds. This new technique will require an additional step at the end of the manufacturing process, and could be integrated as early as 2013.