New proteins enable scientists to control cell activities
© Sailan Shui 2021 EPFL
School of Engineering scientists have developed new controlled proteins and used to switch cellular activities on and off like a light bulb.
Sailan Shui, a doctoral assistant at EPFL’s Laboratory of Protein Design and Immunoengineering, enjoys playing with proteins, activating and deactivating them as she wishes, as if light switches that can be turned on and off. However, instead of using electronic, her method relies on proteins to trigger the process. Shui’s research has just been published in Nature Communications.
To develop her method, Shui and her colleagues began by computationally modeling proteins that don’t exist naturally. She then assembled proteins into OFF- and ON-switches. “The first step was joining the two synthetic proteins together and making sure they can work in tandem. One protein acts like cement, gluing the entire structure together, and the other is a drug receptor. We also had to find two proteins that form strong, stable bonds so that they remain attached,” says Shui.
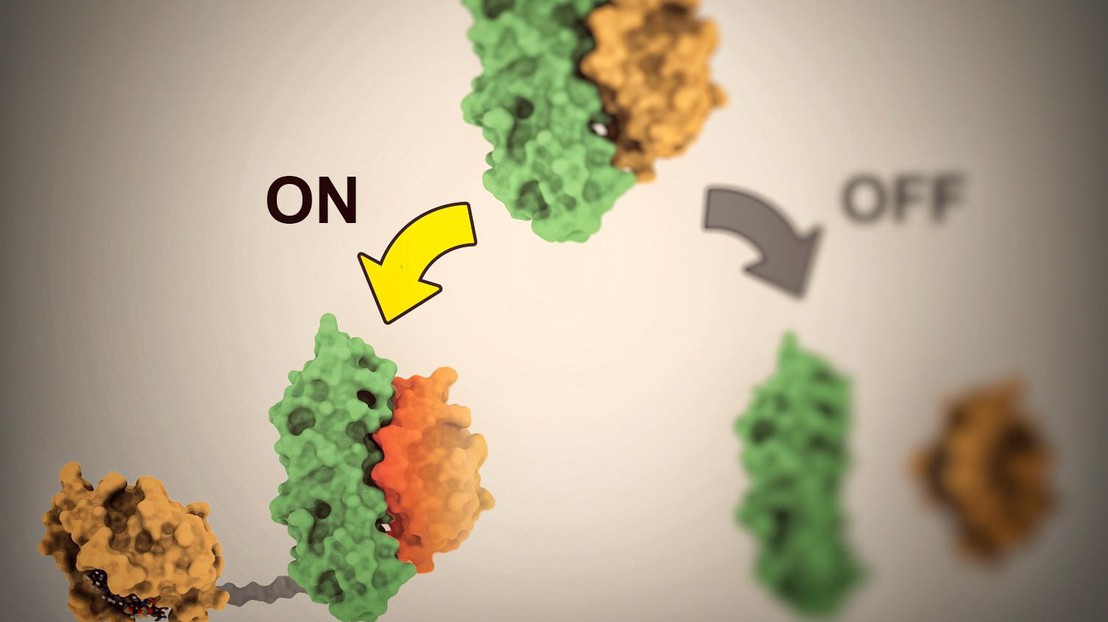
A molecule flips the switch
Once the protein pairs were formed, the next step was to find a third type of molecule able to, alternatively, bind the existing structure and activate it, or break it apart and deactivate it. Shui designed her systems to be responsive to a clinically approved drug Venetoclax. This drug is usually administered to treat cancer, but the scientist discovered a new use for it. “I basically transformed Venetoclax into an on-off switch for the proteins,” says Shui.
When the drug comes into contact with the proteins, it has the ability to either activate them or separate them and consequently deactivate them. “In our method, it’s the Venetoclax molecules that serve as the light switch. They’re the ones that activate or deactivate the proteins,” she says. That means she can control when the proteins are activated, and for how long.
Monitoring cell activity
The hope is that these protein switches can one day be used as intermediaries with cells inside the human body. “We could place the protein switches inside specific cells, for example, so that they can be activated when we want,” says Shui. “That way, when we’re ready, we can apply the stimulus and observe the cellular response.” Bruno Correia, who heads the EPFL lab where Shui is conducting her research, adds: “This type of protein circuit, where the same compound serves two diametrically opposed functions, could be a promising method for monitoring the safety and efficacy of modified cells.”
This work was supported by the Swiss National Science Foundation, the Swiss National Center of Competence for Molecular Systems Engineering, and the Swiss National Center of Competence in Chemical Biology. Shui was supported by a grant from the Biltema and ISREC foundations. The paper’s coauthors who have also received funding are: Correia, the recipient of a Starting Grant from the European Research Council (No. 716058); Leo Scheller, the recipient of a Swiss Cancer League grant; and Pablo Gainza, who is funded in part by an EPFL Fellows grant obtained through an H2020 Marie Sklodowska-Curie action.