New method unlocks cyclopropenes' potential for drug discovery
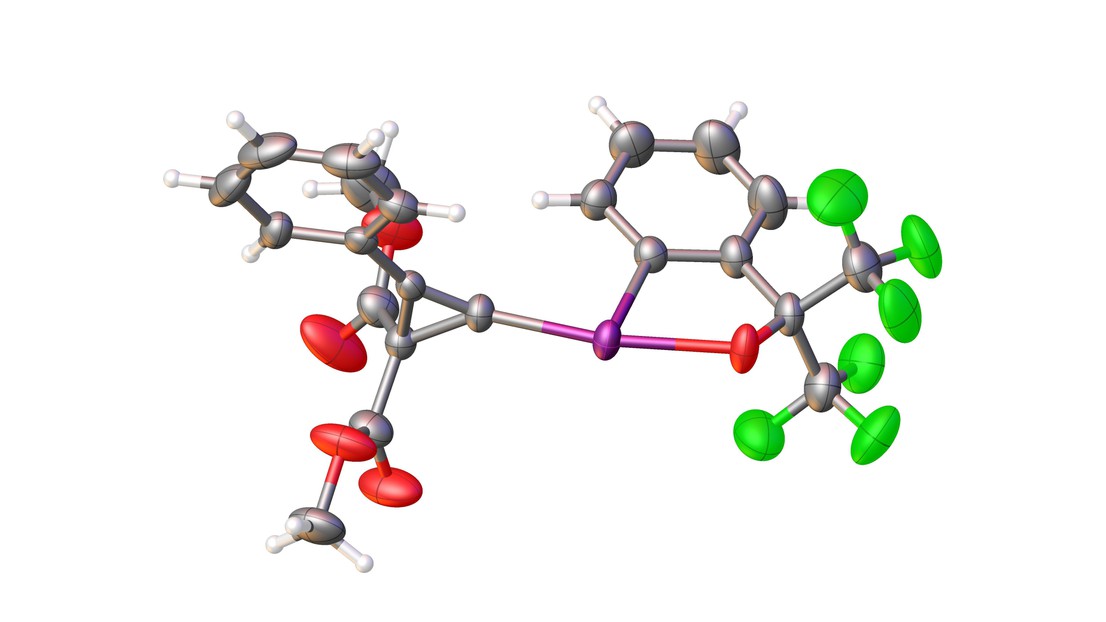
The chemical structure of the reagent. Credit: X. Li (EPFL)
Researchers at EPFL have developed a novel method to introduce cyclopropene, the most strained carbocyclic compound, into organic compounds efficiently under ambient conditions, offering new pathways for drug modification and complex molecular construction.
In the world of chemical synthesis, small changes can have massive impact. Chemists continuously seek novel methods to create complex molecules more efficiently.
One of the most promising approaches, yet highly challenging, involves highly strained carbon rings, such as cyclopropenes. “Highly strained” is a term that describes molecules that have a lot of internal energy due to their unusual bond angles and ring structures, making them very reactive but also unstable.
Cyclopropenes are the smallest unsaturated carbocycles with significant strain energy, which makes them highly reactive, a valuable trait for chemical reactions, especially for synthesizing complex molecules. Their small size and rigid three-membered ring structure make cyclopropenes ideal for creating highly functionalized compounds with well-defined tridimensional structures, which has been recognized as an essential feature for the development of drugs, chemical probes and materials displaying enhanced properties.
But cyclopropenes are notoriously unstable, which has made them difficult to use in practical applications. Meanwhile, traditional methods to incorporate these molecules into other compounds often fail due to their tendency to decompose. Consequently, finding a reliable way to transfer cyclopropene moieties to target molecules has been a longstanding goal in synthetic chemistry.
Now, researchers led by Professor Jérôme Waser at EPFL have developed an efficient strategy to transfer cyclopropene moieties to target molecules and to do so under ambient conditions.
The scientists began by developing a new class of reagents for the transfer of cyclopropene fragments displaying higher stability, but without jeopardizing reactivity. They did this by using “hypervalent iodine reagents”, which can be accessed in one step from cyclopropenes themselves and can be easily stored and manipulated.
“The stable cyclopropene reagents react under ambient conditions in the presence of a commercially available gold catalyst with substrates such as terminal alkynes or vinyl boronic acids, broadly available building blocks used in the synthesis of complex molecules, including pharmaceuticals,” says Waser. The process itself is operationally simple, tolerant to numerous reactive chemical groups, and can be applied to the late-stage modification of complex natural products and drugs.
The researchers went a step further and used their new stable cyclopropene reagents to modify drug molecules, such as artesunate, an anti-malarial medication bearing sensitive functionalities, which remains intact under the developed conditions resulting in a clean and complete cyclopropene transfer. These results demonstrated the new method's robustness and efficiency, and its potential for creating new pharmaceuticals and enhancing the properties of existing ones by modifying their molecular structures more easily.
The team also showed that their method can be extended to other complex natural products and bioactive molecules. The ability to attach cyclopropene moieties reliably under ambient conditions means that chemists can now explore new reactions and build complex rigidified molecules that were previously difficult or impossible to synthesize.
“The technique disclosed in this study opens the gateway for cyclopropene-centered synthetic campaigns,” says Xiangdong Li, the study’s first author. “Researchers from synthetic chemistry, as well as from chemical biology, medicinal and material chemistry could all benefit from this technique. The equivalence strategy presented in this study not only provides an access to harness the most strained reaction intermediates for synthetic chemistry, but also should serve as an inspiration for others to explore and exploit synthetically useful high-energy reagents in the future.”
EPFL
Xiangdong Li, Matthew D. Wodrich, Jérôme Waser. Accessing elusive σ-type cyclopropenium cation equivalents through redox gold catalysis. Nature Chemistry 23 May 2024. DOI: 10.1038/s41557-024-01535-8