New immunotherapy becomes activated specifically in tumors
Un médicament commutable pour une immunothérapie plus sûre © 2021 EPFL
EPFL scientists have developed a chemical method for targeting the effects of cancer-fighting immunotherapy drugs only to the tumor tissue, making the drugs less toxic to the rest of the human body.
Immunotherapy drugs are promising new weapons in the fight against cancer, but they are so strong that they can be toxic to the rest of the human body. The basic idea behind immunotherapy drugs is simple. Doctors inject special kinds of drugs, especially proteins such as antibodies and cytokines prepared or modified in a lab, into a patient, where they activate the patient’s immune cells –T-cells, NK cells, and so on – and help these cells fight the tumor. In short, immunotherapy drugs work like a powerful cocktail that boosts a patient’s own immune system.
“After being prescribed by a doctor, immunotherapy drugs are administered intravenously,” says Li Tang, head of the Laboratory of Biomaterials for Immunoengineering at EPFL’s School of Engineering. “Once inside the body, the drugs spread all over – not just where the tumor or any metastases are located. The problem is that the proteins in the drugs are so strong that they damage healthy tissue.” Many of the immunotherapy treatments already out there have proven to be highly effective against cancer in preclinical studies. But they often can’t be used to save people because they’re too toxic to the rest of the body. “The treatments that are used in patients today have been toned down so they’re less potent,” says Tang. “That makes them safer, but also less effective at destroying tumors. Our aim is to keep all the potency of immunotherapy, because it will be an important treatment option for cancer patients.”
Tang and his team therefore developed a method whereby the immunotherapy proteins are activated only when they come into the tumor tissues. The EPFL team is one of the pioneers to develop this kind of technology through a universal chemical approach. “We were able to achieve this thanks to our cross-disciplinary approach,” says Tang. “Our method draws on techniques from both chemistry and immune engineering.” The research has just been published in Science Advances.
A special environment
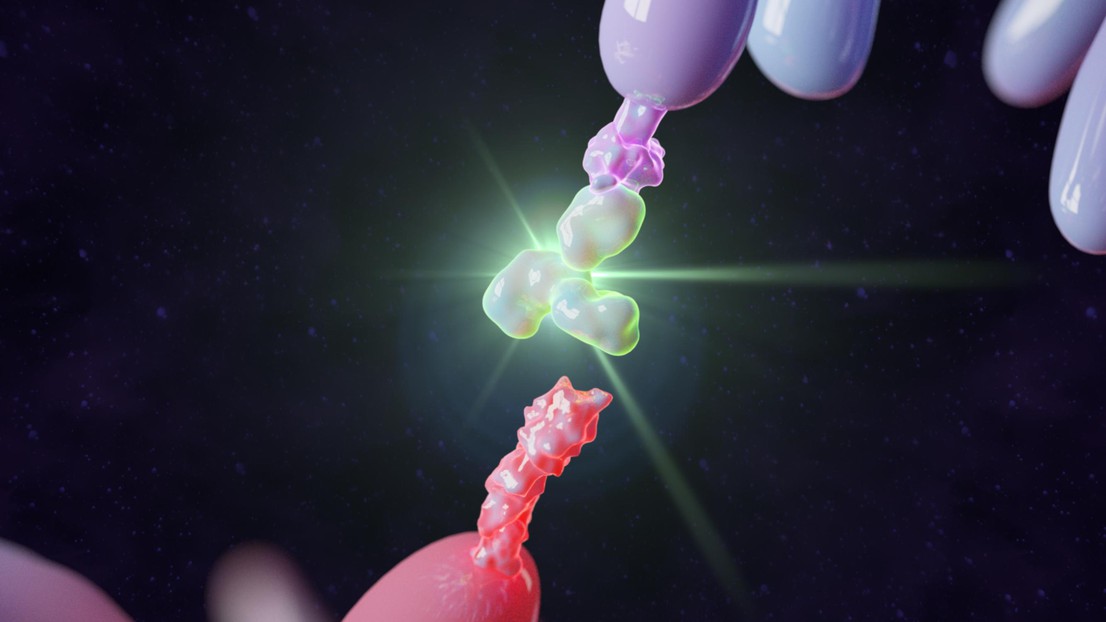
Yu Zhao, a postdoc at Tang’s lab, began by utilizing the chemical properties around tumors. “The tumor microenvironment is different from the rest of the body. The pH is lower, meaning it’s more acidic, and it has a high reducing potential,” he says. Zhao used these facts, already known to scientists, to develop a kind of polymer shield for the protein drugs that would let them travel harmlessly through the body until they reach the tumor.
“To create the shield, I first developed stimuli-responsive chemical bonds that attach to the surface of the protein molecules, like little hooks,” says Zhao. “Then I took polymers – which are long chains of molecules – and ‘hooked’ them to the bonds on of the protein molecules. Once attached to the protein surface, the polymers wrap around them, like a protective shield.”

That shield is designed to break down when exposed to the unique chemical environment in the tumor tissue. Tang explains: “Chemical reactions in the tumor microenvironment break the bonds at the protein surface, thereby removing the polymer shield. The protein drugs are then free to activate the patient’s cancer-fighting lymphocytes selectively in the tumor tissue.”
It took Zhao several years of research and countless experiments to find the right chemical combination for the new method. And it will probably take another several years, along with a sizeable amount of funding, before the method is potentially used clinically to treat cancer.
This work was supported in part by the Swiss National Science Foundation (SNSF Project grant 315230_173243), ISREC Foundation with a donation from the Biltema Foundation, Swiss Cancer League (No. KFS-4600-08-2018), the European Research Council under the ERC grant agreement MechanoIMM (805337), Kristian Gerhard Jebsen Foundation, Foundation Pierre Mercier pour la science, Anna Fuller Fund Grant, and EPFL. Y.Z. is supported by the Eurotech Postdoctoral Programme that is cofounded by the European Commission under its framework programme Horizon 2020 (grant agreement 754462). M.G. is supported by the Chinese Scholarship Council (CSC, No. 201808320453). S.V.H. is supported by a Ghent University – Special Research Found (BOF PDO.2009.0006.01) postdoctoral fellowship and FWO travel grant (V412920N).

