New cancer models could help personalize lymphoma treatments
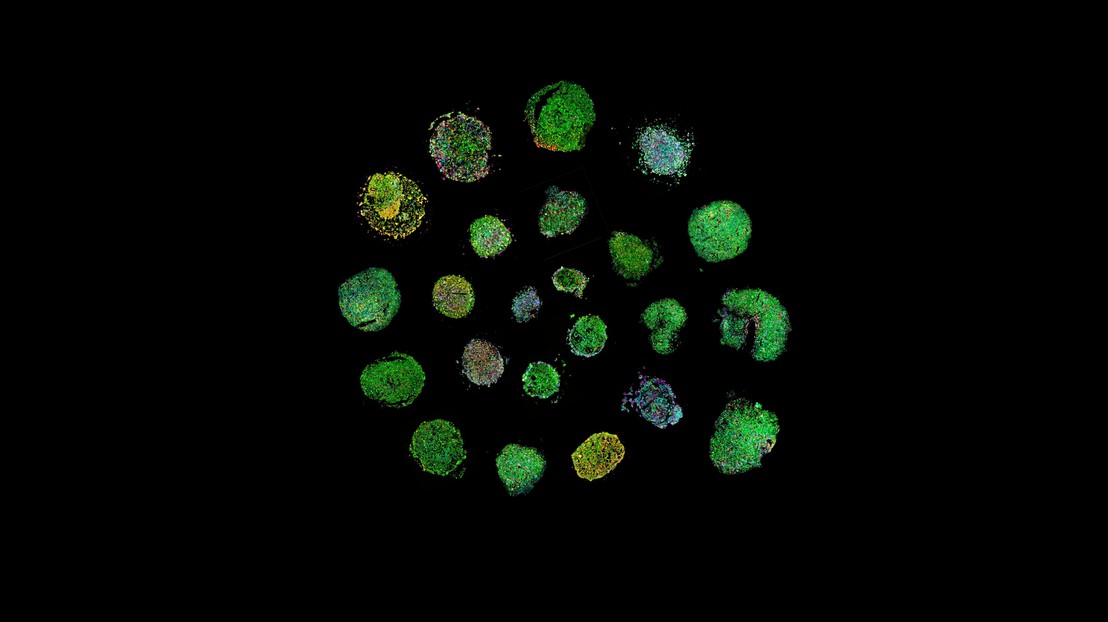
Lymphomoid spiral (created by Albert Santamaria-Martinez).
Scientists at EPFL have developed "lymphomoids," a pioneering cancer model that preserves the structure and multicellular composition of lymphoma tumors in the lab. Lymphomoids offer an innovative way to test the efficacy of lymphoma treatments and better predict individual responses.
Cancer is notoriously complex, with each tumor responding to different therapies. This is also true of lymphomas, a type of blood cancer that originates from lymphocytes, a subgroup of immune cells that help fight infections. Researchers constantly seek ways to identifyied the best therapy for each patient.
Traditional methods for testing the efficacy of therapies in lymphoma are limited. For example, patient-derived xenograft models, where human tumors are grown in mice, are effective but also slow and expensive. Moreover, they don’t fully capture the diversity of interactions between the tumor and immune cells.
More recently, scientists started developing “tumor avatars”— these represent new systems to maintain cells or tissue samples outside of the patient’s body (ex vivo). Tumor avatars are quite promising tools. However, it was particularly difficult to maintain the original structure and cell composition in lymphomas.
To address these challenges, Albert Santamaria-Martínez and Elisa Oricchio at EPFL, in close collaboration with clinicians at CHUV, developed an advanced ex vivo model called “lymphomoids.” They identified a specific environment that allows to maintain fragments of lymphoma tissue ex vivo for several days. In these conditions, they were able to preserve the tumor’s architecture, cellular diversity, and microenvironment.
The researchers collected 27 human lymphoma samples and demonstrated by using imaging-based analyses and spatial molecular profiles that lymphomoids preserve the phenotypic and molecular characteristics of the original tumors.
In a study involving various types of human B-cell lymphomas, lymphomoids were kept alive and structurally intact for several days, allowing researchers to examine how the samples responded to different drugs. The team tested a range of cancer drugs on the lymphomoids, including Bruton's tyrosine kinase (BTK) inhibitors, PI3K inhibitors, and BCL-2 inhibitors, to determine whether these treatments could reduce the growth and proliferation of lymphoma cells.
Lymphomoids showed a range of sensitivities to cancer drugs, closely mirroring the clinical responses of patients whose tissue samples were used. For instance, lymphomoids from a patient whose tumor responded well to BTK inhibitors also showed sensitivity to the same drug in the ex vivo model. In another case, lymphomoids from a patient who was resistant to lenalidomide demonstrated a similar resistance during ex vivo testing.
This suggests that lymphomoids can serve as a reliable tool for predicting how individual patients might respond to specific treatments. By allowing researchers to test drug effectiveness on patient-derived samples, lymphomoids offer a promising new way to personalize cancer treatment. They could eventually enable doctors to use a patient’s tissue sample to find the most effective treatment for that individual before beginning therapy, potentially sparing patients from unnecessary treatments and side effects.
Additionally, lymphomoids could also be used in clinical trials to test emerging cancer therapies and explore the complex dynamics between tumor cells and immune cells during treatment.
Other contributors
- Swiss Cancer Center Léman
- Centre Hospitalier Universitaire Vaudois (CHUV)
- University of Lausanne (UNIL)
- Swiss Institute of Bioinformatics (SIB)
- Institut Central des Hôpitaux (ICH)
Personalized Health and Related Technologies (PHRT)
ISREC foundation
Fondation Aclon
Accentus Foundation
Fondazione San Salvatore
Albert Santamaria-Martínez, Justine Epiney, Divyanshu Srivastava, Daniele Tavernari, Marco Varrone, Dina Milowich, Igor Letovanec, Thorsten Krueger, Rafael Duran, Giovanni Ciriello, Anne Cairoli, Elisa Oricchio. Development of patient-derived lymphomoids with preserved tumor architecture for lymphoma therapy screening. Nature Communications 09 December 2024. DOI: 10.1038/s41467-024-55098