LIMNO Reports New Route to Make Stable Organic Solar Cells
© 2019 EPFL/K.Sivula
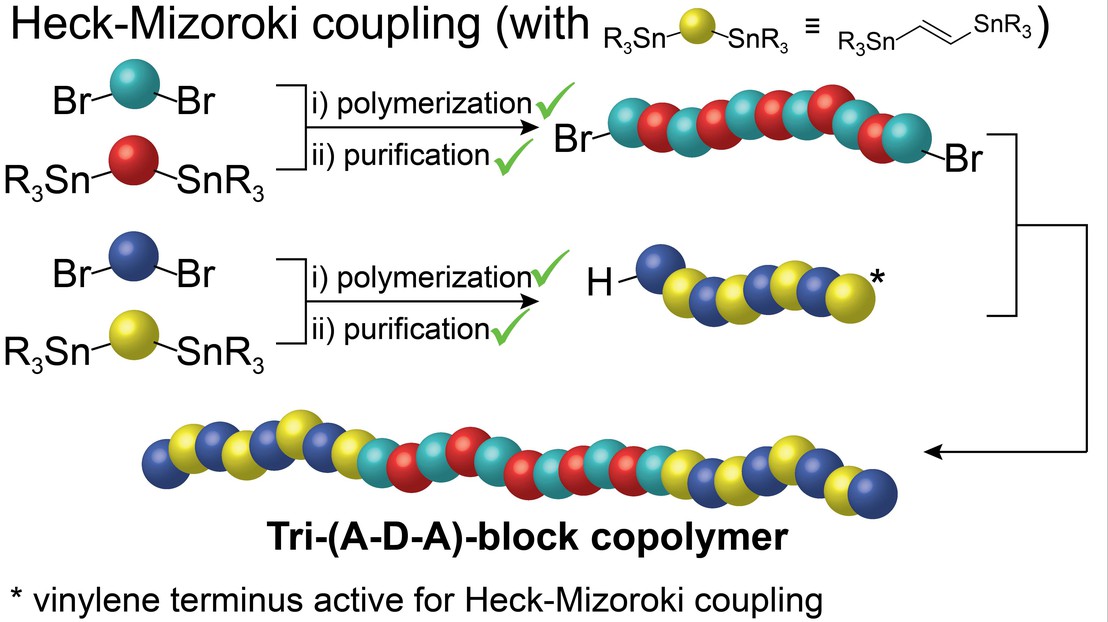
The stability of donor-acceptor bulk heterojunction organic photovoltaics (BHJ OPV) can be increased by using fully conjugated block co-polymers (BCPs) but the lack of facile routes to prepare BCPs from diverse monomers has limited the advancement of BCP based BHJ OPVs. LIMNO has advanced the field in a new report.
In a paper published in ACS Macro letters we introduce a synthetic strategy for step-growth BCPs employing 1,2-bis(trialkylstannyl)ethene as one monomer, which, in addition to offering improved backbone planarity, directly yields a vinylene-terminated macromonomer suitable for Heck-Mizoroki coupling. The benefits of our strategy, which facilitates the preparation of functionalized macromonomers suitable for BCP synthesis, is demonstrated with a representative BCP based on a diketopyrrolopyrrole (DPP) copolymer coded pBDTTDPP as the donor block and a perylenediimide (PDI) copolymer coded as pPDIV as the acceptor block. Feed ratio optimization affords control over the macromonomer chain-end functionalities and allows for the selective formation of a tri-BCP consisting of pPDIV-b-pBDTTDPP-b-pPDIV, which is employed in a single-component BHJ OPV. Devices achieved a power conversion efficiency of 1.51% after thermal stress at 150°C compared to 0.02% for control device consisting of a comparable blend of pBDTTDPP and pPDIV. The difference in performance is ascribed to the morphological stability of the BHJ when using the BCP.