Interfacial engineering using crown ethers in perovskite solar cells
© 2024 EPFL
Interfacial engineering through lead binding (crown ethers) achieves lead ion sequestration and robust interfacial passivation, enhancing stability and efficiency of perovskite solar cells.
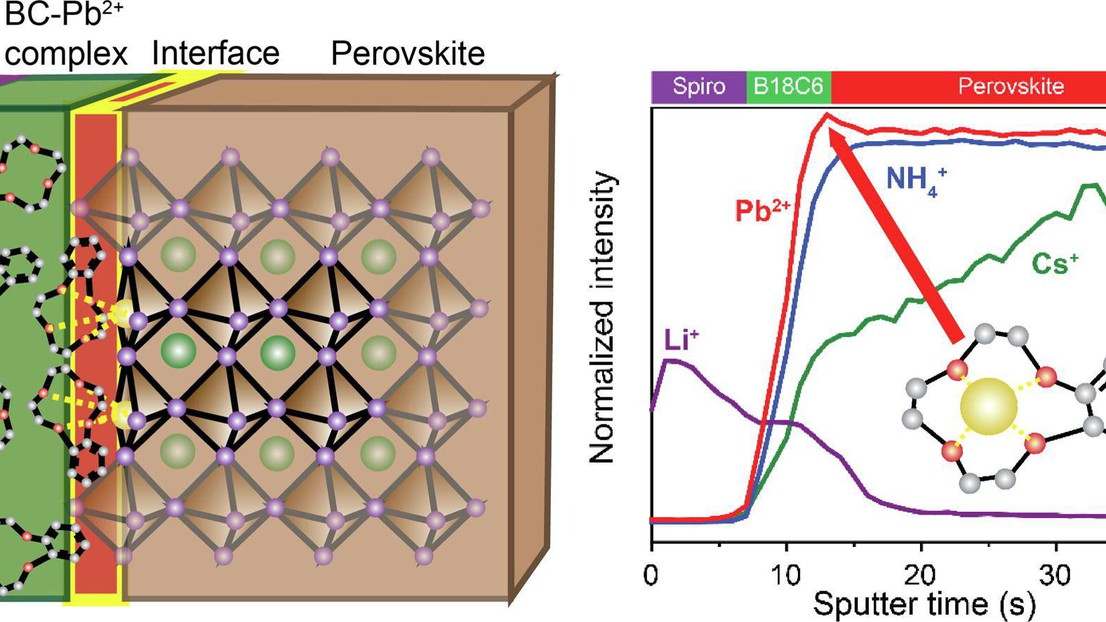
In a collaboration between LIMNO and NEMD lab we have pioneered an approach that not only rectifies lead leakage but also places paramount importance on the attainment of rigorous interfacial passivation in Perovskite solar cells (PSCs). Crown ethers, notably benzo-18-crown-6-ether (B18C6), were strategically integrated at the perovskite-hole transport material interface. Crown ethers exhibit a dual role: efficiently sequestering and immobilizing Pb2+ ions through host-guest complexation and simultaneously establishing a robust interfacial passivation layer. Selected crown ether candidates, guided by density functional theory (DFT) calculations, demonstrated proficiency in binding Pb2+ ions and optimizing interfacial energetics. Photovoltaic devices incorporating these materials achieved exceptional power conversion efficiency (PCE), notably 21.7% for B18C6, underscoring their efficacy in lead binding and interfacial passivation. Analytical techniques, including time-of-flight secondary ion mass spectrometry (ToF-SIMS), ultraviolet photoelectron spectroscopy (UPS), time-resolved photoluminescence (TRPL), and transient absorption spectroscopy (TAS), unequivocally affirmed Pb2+ ion capture and suppression of non-radiative recombination. Notably, these PSCs maintained efficiency even after enduring 300 h of exposure to 85% relative humidity. This research underscores the transformative potential of crown ethers, simultaneously addressing lead binding and stringent interfacial passivation for sustainable PSCs poised to commercialize and advance renewable energy applications.