How the microbiome drives the evolution of immune defenses
A sketch of Drosophila fruit flies. 2023 EPFL/ Hanson et al. DOI: 10.1126/(science.adg5725)- CC-BY-SA 4.0
A new study from researchers at EPFL reveals how bacteria shape the immune system of fruit flies, shedding light on the evolution of host defenses against specific pathogens and beneficial microbes.
Animals and humans coexist with a vast array of microorganisms known as the microbiome, forming an intricate relationship that can range from mutually beneficial to pathogenic. To safeguard against harmful pathogens and maintain the presence of beneficial microorganisms, animals have evolved various defenses.
One of those are the small antimicrobial peptides (AMPs); small peptides that combating invading microbes. AMPs are crucial immune effectors in both plants and animals, fighting against potential infections while also influencing the composition of the host's microbiome.
While previous studies have shown that AMPs evolve rapidly, little was known about the driving forces behind this evolution. For example, different animals have different “repertoires” of AMP genes, while lacking others found elsewhere. Understanding the evolutionary “logic” behind this is important not just as an ecological study, but also for the development of innovative strategies to prevent infections by targeting specific microbial threats.
Now, a study led by three scientists at EPFL uncovers the selective pressures driving the evolution of AMPs and how they control bacteria in the host’s microbiome. The work was carried out by Bruno Lemaitre’s group at EPFL’s School of Life Sciences, led by Mark Hanson (now at the University of Exeter) and Lena Grollmus. It is published in Science.
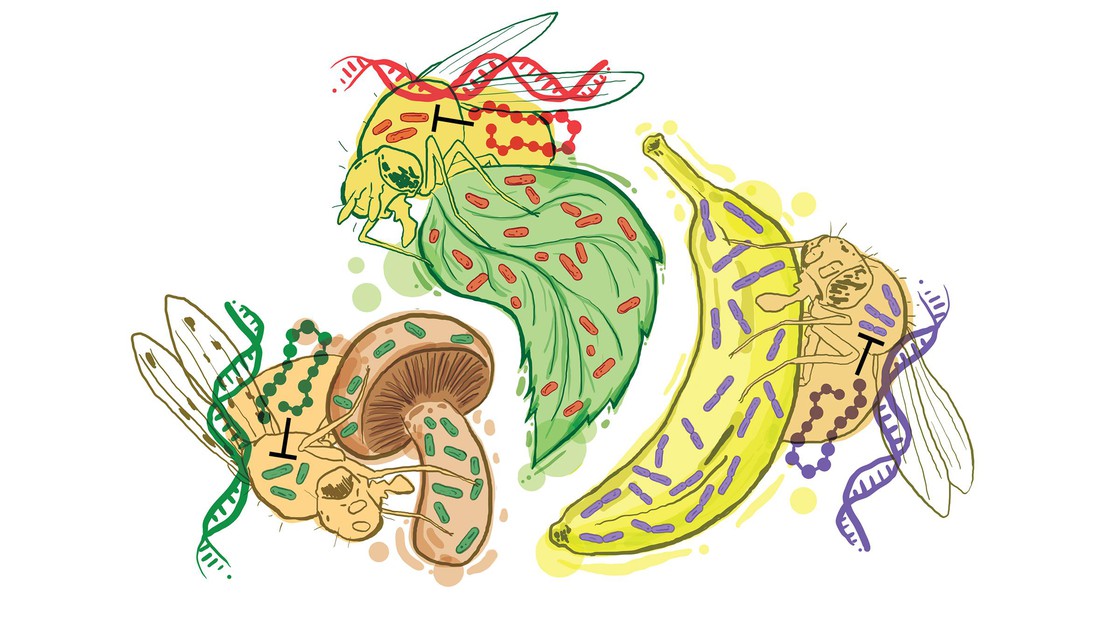
The researchers focused on Diptericin (Dpt), a small antimicrobial peptide that mainly defends flies against Gram-negative bacteria, disrupting their bacterial membrane. Looking at the fruit fly Drosophila, the team examined how Diptericins function and evolve in response to their microbial environment. The team discovered that different types of Diptericins, known as DptA and DptB, play specific roles in the fruit fly's defense against different bacteria.
By screening Drosophila mutants lacking specific AMP gene families, the researchers found that DptA is effective against Providencia rettgeri, a natural pathogen of Drosophila. Meanwhile, DptB helped the host resist infection by multiple species of Acetobacter, some of which reside in Drosophila’s gut and help its physiology and development. In contrast, DptA played no significant role against Acetobacter and DptB played no significant role against Providencia.
Analyzing the evolutionary history of the Diptericin genes, the scientists found two instances of convergent evolution that lead to DptB-like genes in fruit flies that feed on fruit, an environment associated with high levels of Acetobacter. This suggests that DptB evolved to control Acetobacter in the ancestral fruit-feeding Drosophila.
The study also found that fruit flies with different ecological niches, such as mushroom-feeding or being plant-parasites, had either lost the DptB gene or both DptA and DptB genes, corresponding to an absence of Acetobacter or both Providencia and Acetobacter, respectively.
Meanwhile, variations in DptA and DptB sequences were found to predict the host’s resistance to infection by these bacteria throughout the Drosophila genus. This highlights the evolutionary adaptation of the fly's immune repertoire to combat specific microbes prevalent in its surroundings.
To validate their findings, the researchers infected various Drosophila species with different variants of DptA and DptB genes. The results were striking: the resistance of the host to infection by P. rettgeri and Acetobacter was readily predicted just by the presence and polymorphism of the DptA or DptB genes, even across fly species separated by almost 50 million years of evolution.
The work sheds light on the dynamics that shape the host’s immune system and how the host’s defenses adapt to combat specific pathogens while fostering beneficial microorganisms. The findings propose a new model of AMP-microbiome evolution, incorporating gene duplication, sequence convergence, and gene loss, all guided by the host's ecology and microbiome. This model explains why different species possess specific repertoires of AMPs, offering insights into how host immune systems rapidly adapt to the suite of microbes associated with a new ecological niche.
“The way our bodies fight infections is very complex,” says Mark Hanson. “But this sort of research helps us to view our immune system in a new light. It helps us ask: ‘why is our immune system made the way it is?’ That can help us learn how to fight infections, including ones that resist antibiotics.”
Swiss National Science Foundation (SNSF)
Novartis Foundation
M.A. Hanson, L. Grollmus, B. Lemaitre. Ecology-relevant bacteria drive the evolution of host antimicrobial peptides in Drosophila. Science 20 July 2023. DOI: 10.1126/science.adg5725