How a key immune protein is regulated in the cell
EPFL/ iStock
Scientists at EPFL have determined how a protein that is critical in our first line of immune defense is regulated in the cell to prevent autoinflammatory diseases.
How does a cell “know” that it’s infected? This is a key question for innate immunity, our first line of defense to any infection or injury, made up of cells that quickly identify pathogens, like viral DNA. To do this, the cells use receptors that can identify nucleic acids — the building blocks of DNA — that in turn activate a signaling molecule called STING (for Stimulator of interferon genes).
In a cascade of molecular “domino” – what scientists call a “signaling pathway” – STING begins to work after the enzyme cyclic GMP-AMP synthase, or cGAS, so the complete signaling pathway is known as cGAS-STING. Its role is to detect foreign DNA, e.g. from bacteria or viruses, that has invaded the cell.
When foreign DNA invades the cell, the cGAS-STING signaling pathway turns on. STING exits the cell’s endoplasmic reticulum where proteins are synthesized, and moves to the Golgi apparatus, where proteins undergo modifications and “final touches” before being packaged and sent to their target destination.
In the Golgi, an enzyme attaches a few phosphate groups to STING – a common mechanism known as “phosphorylation” that energizes proteins in the cell. STING then begins to activate genes that turn on the cell’s defense mechanisms to fight the infection.
Given how central STING is to a critical function like innate immunity there has been a flurry of research on it, particularly by the group of Andrea Ablasser at EPFL’s School of Life Sciences. Nonetheless, little is known about how STING is regulated and how it actually stops turning on genes – a key question considering that STING can lead to some serious autoinflammatory diseases when it doesn’t work.
In a new study published in Nature, Ablasser’s group have now identified the protein that terminates the activity of STING. The protein, named adaptor protein complex-1 (AP-1) packages STING into vesicles, which are small enclosed pods made of a lipid bilayer like the one that forms the cell’s membrane.
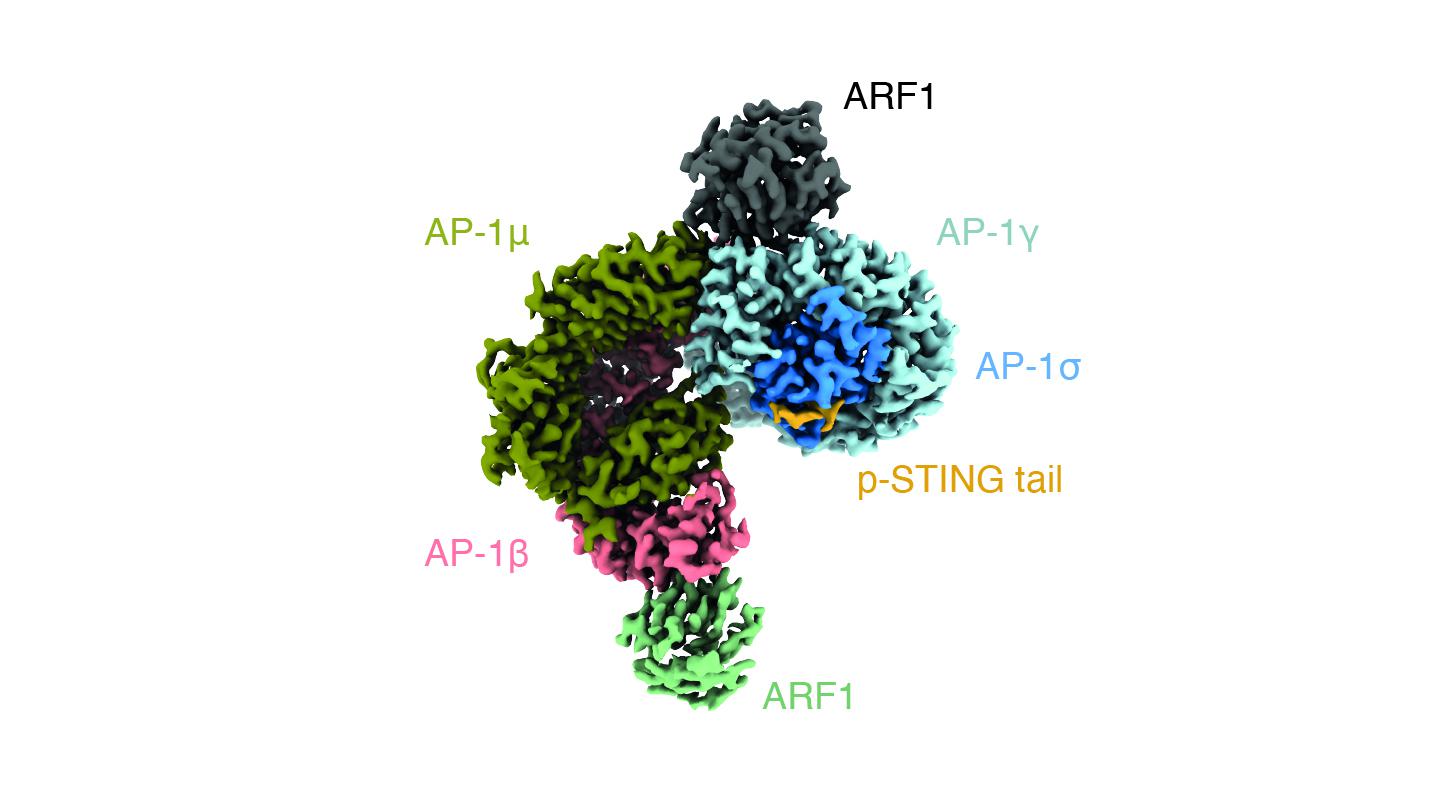
Vesicles usually transport materials in and out of the cell, for example in endocytosis and secretion respectively. Like most vesicles in the cell, the ones in which AP-1 packages STING are coated with clathrin (from the Latin clathrus = lattice), a protein that forms a three-legged shape and attaches itself on the outside of the vesicle’s surface.
The researchers found that AP-1 recognizes a specific pattern at the cytosol-side end of the STING protein; specifically, two leucine amino acids that makes AP-1 engage the protein. By using cryo-electron microscopy, the scientists were able to determine the structure of AP-1 and show that it regulates the phosphorylation of STING, thereby turning it on and off.
Further confirming their findings, the team also showed that when AP-1 is suppressed, STING-induced immune responses become worse. The authors conclude: “Our results explain a structural mechanism of negative regulation of STING and establish that signalling initiation is inextricably associated with its termination to enable transient activation of immunity.”
Other contributors
University of California, Berkeley
Swiss National Science Foundation
Dr. Josef Steiner Cancer Research Foundation,
European Union’s Horizon 2020 Research and Innovation program
Leenaards Foundation
Fondation Acteria
U.S. National Institutes of Health
EMBO Post-doctoral Fellowships
Ying Liu, Pengbiao Xu, Sophie Rivara, Chong Liu, Jonathan Ricci, Xuefeng Ren, James H. Hurley, Andrea Ablasser. Clathrin-associated AP-1 controls termination of STING signalling. Nature 19 October 2022. DOI: 10.1038/s41586-022-05354-0