Flowing fluids shape the social life of gut microbes
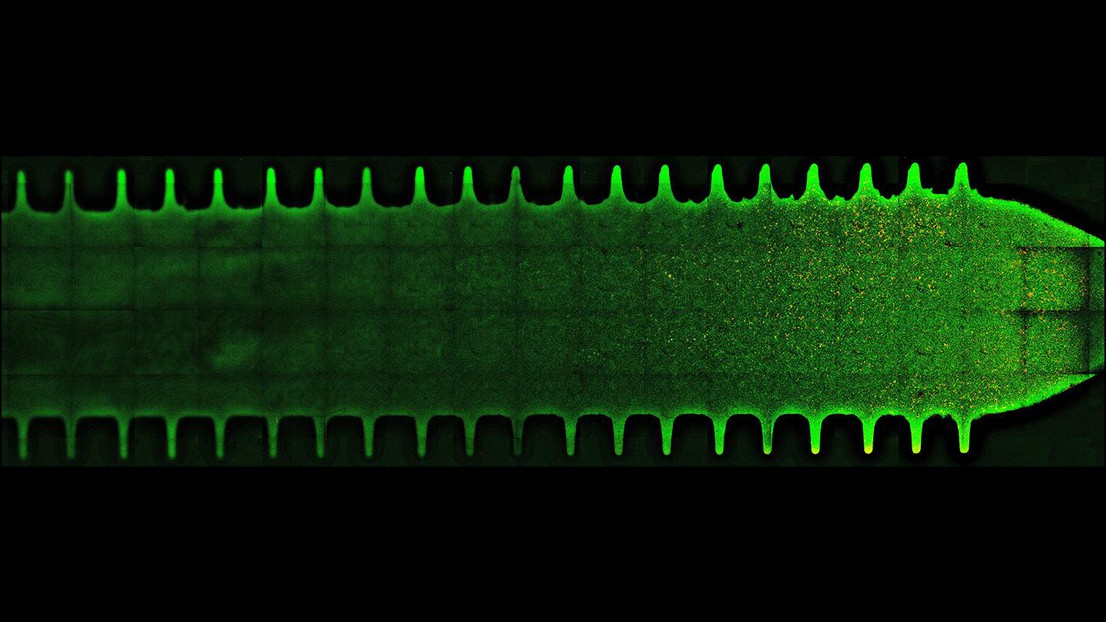
The gut commensals Bacteroides thetaiotaomicron and B. fragilis form biofilms in crypt-like features. Credit: Jeremy Wong (EPFL)
A groundbreaking study reveals that the flow of fluids influences the spatial organization of bacterial communities that inhabit our intestines, revealing an overlooked factor potentially mediating our microbiome and gut health.
Our gut is home to a diverse community of bacteria known as the gut microbiota. These bacteria play a crucial role in our health by supporting digestion, producing essential nutrients, maintaining a balanced immune system and even affecting our mood and behavior.
Understanding the factors that shape gut microbiota composition is crucial, as disruptions in this delicate balance have been associated with various health conditions, including inflammatory bowel disease, obesity, and metabolic disorders.
In a new study, scientists led by Alex Persat at EPFL’s School of Life Sciences now provides new insights into how the physical forces of flowing fluids in our gut shape bacterial communities. Working with the lab of Tom Battin at EPFL’s School of Architecture, Civil and Environmental Engineering, and colleagues at ETH Zurich, the work provides insights into the intricate mechanisms by which different bacterial species of the microbiota interact with one another by sharing nutrients.
Wanting to understand the role of fluid flow in shaping interactions between gut bacteria species, the researchers investigated two common gut bacteria known to be beneficial to gut health as representatives: Bacteroides thetaiotaomicron and Bacteroides fragilis.
The team looked at how the two species share nutrients when exposed to dextran, a common food additive. They grew the bacterial communities in a microfluidic device under anaerobic conditions, which simulated the intestinal environment in the lab. Under these conditions, the bacterial communities grew in the form of multicellular communities called biofilms, where nutrient sharing impacts the position of different species relative to one another.
The researchers then visualized the populations with high-resolution microscopy, and for the first time, were able to understand the physical principles guiding the organization of microbiota communities.
The study showed that the flow of fluids within the gut influences how these bacteria interact and exchange beneficial by-products of dextran metabolism, forming biofilms attached to the inner surface of the intestine.
In addition, the exchange of these public goods also shapes the spatial distribution of the community. “Fluid flows that resemble the ones these bacterial species experience in the human colon strongly impact the spatial organization and composition of syntrophic communities via distribution of public goods,” says Jeremy Wong, the study’s first author.
The study also found that the strength of the flow also impacts the formation of biofilm by the B. fragilis bacteria: When the flow was too strong, the concentration of beneficial by-products at the surface decreased, limiting the growth of B. fragilis biofilms. This suggests that physical factors can impact the overall composition and stability of gut bacteria communities.
The research highlights the importance of considering not only the chemistry but also the physical forces at play in shaping gut bacteria communities. This additional perspective could unlock novel approaches of promoting a healthy gut microbiome and prevent or even treat diseases that arise from problems among bacterial communities.
Jeremy Wong is a PhD student supervised by Alex Persat and Tom Battin as part of the iPhD program of EPFL’s School of Life Sciences.
Swiss National Science Foundation
EPFL iPhD program
Jeremy P. H. Wong, Michaela Fischer-Stettler, Samuel C. Zeeman, Tom J. Battin, Alexandre Persat. Fluid flow structures gut microbiota biofilm communities by distributing public goods. PNAS 12 June 2023; 120 (25) e2217577120. DOI: 10.1073/pnas.2217577120