Facile CO Cleavage by a Multimetallic CsU2 Nitride Complex
© WILEY-VCH Verlag GmbH & Co
Marta succeeded in breaking CO (the molecule with the strongest chemical bond) using an uranium nitride.
Her work was just published in ACIE She is now looking to way to implement this reactivity in catalytic cycles.
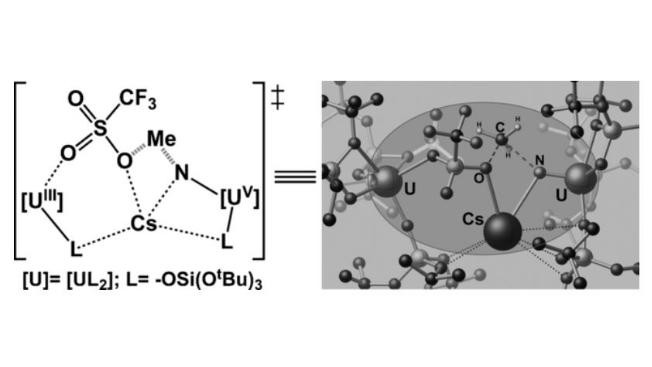
Uranium nitrides are important materials with potential for application as fuels for nuclear power generation, and as highly active catalysts. Molecular nitride compounds could provide important insight into the nature of the uranium–nitride bond, but currently little is known about their reactivity. In this study, we found that a complex containing a nitride bridging two uranium centers and a cesium cation readily cleaved the C≡O bond (one of the strongest bonds in nature) under ambient conditions. The product formed has a [CsU2(μ-CN)(μ-O)] core, thus indicating that the three cations cooperate to cleave CO. Moreover, the addition of MeOTf to the nitride complex led to an exceptional valence disproportionation of the CsUIV–N–UIV core to yield CsUIII(OTf) and [MeN=UV] fragments. The important role of multimetallic cooperativity in both reactions is illustrated by the computed reaction mechanisms.