Discovery of a novel aggregation domain in the Huntingtin protein.
© 2012 EPFL
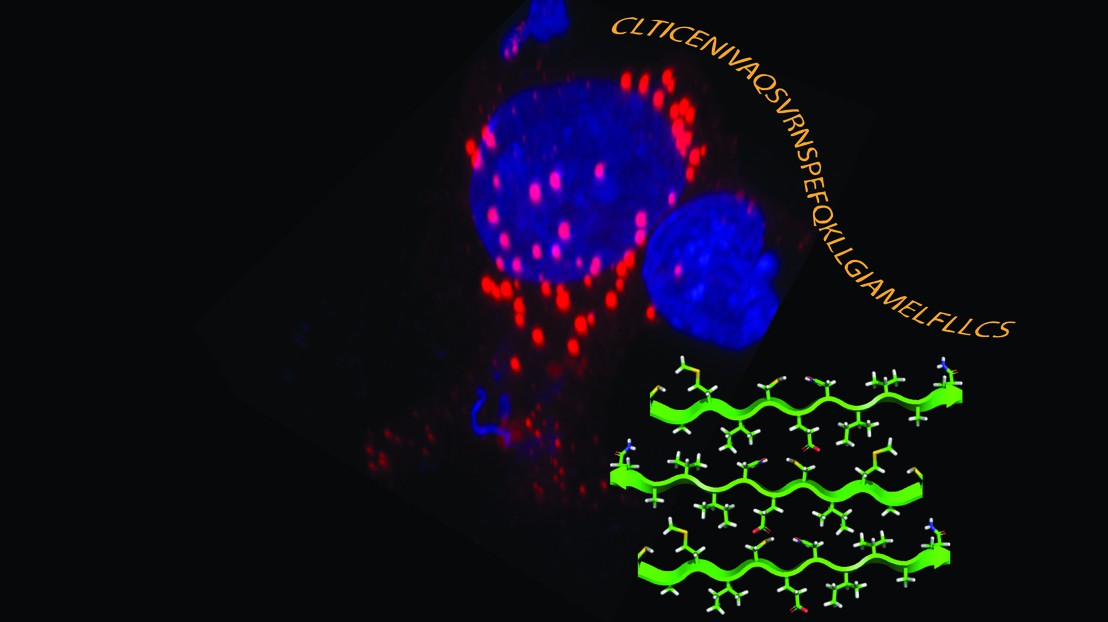
Discovery of a Novel Aggregation Domain in the Huntingtin Protein: Implications for the Mechanisms of Htt Aggregation and Toxicity.
Researcher from the lab of Prof. Hilal Lashuel (Laboratory of Molecular Neurobiology and Neuroproteomics) describe for the first time the discovery of a novel aggregation domain in the Huntingtin protein. The significance of this work is that until now it has been thought that the polyQ domain is the main aggregation domain and key determinant of Htt aggregation. Their studies suggest that this new domain plays an important role in regulating the production, aggregation and toxicity of the toxic N-terminal fragments that are thought to play a key role in the pathogenesis of Huntington’s disease (HD). Follow up studies to validate this hypothesis in cellular and animal models are ongoing in the lab and the group is confident that this work could lead to the identification of novel therapeutic targets for the treatment of HD.