Dimitris N. Chorafas Foundation Award 2019 – Marta Falcone

© 2019 EPFL - Marta Falcone
Small Molecule Activation by Multimetallic Uranium Complexes
EPFL thesis n°9539 (2019)
Thesis director: Prof. M. Mazzanti
"For her ground-breaking research on fundamental uranium chemistry and small molecule activation. She developed original systems for the reduction and functionalization of dinitrogen under ambient conditions. This work opens new perspectives for the sustainable conversion of dinitrogen into higher value products."
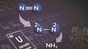
Uranium nitrides are attractive candidates for stoichiometric and catalytic nitrogen transfer reactions, small molecule transformation, and for advanced nuclear fuels. The N-C bond formation reactions are key steps in the construction of value-added chemical compounds. Recently we reported remarkable reactivity studies in f-elements chemistry. Particularly desirable are the synthetic methods using cheap and available C1 feedstock such as carbon dioxide or carbon monoxide. Tert-butoxysiloxides were shown to be very effective ligands in supporting the reactivity of low valent f-elements with small molecules. In particular, it was recently reported the reactivity of the previously described nitride bridged diuranium(IV) complex [Cs{[U(OSi(OtBu)3)3]2(μ-N)}] with CO2, CS2, and CO, yielding to diverse N-functionalized products. Recent results showed that increasing the electron density by reducing the metal centers, leads to a multimetallic K3UNU nitride bridged diuranium(III) complex which shows extraordinary reactivity towards the dinitrogen molecule. The reduction and further functionalization of dinitrogen is achieved by addition of H2 and CO. By changing the linker atom to an oxo bridge to give a K2UOU oxide bridged diuranium(III) complex, a comparative study can reveal a different nature in the M-L-M binding.