Demystifying the immortality of cancer cells
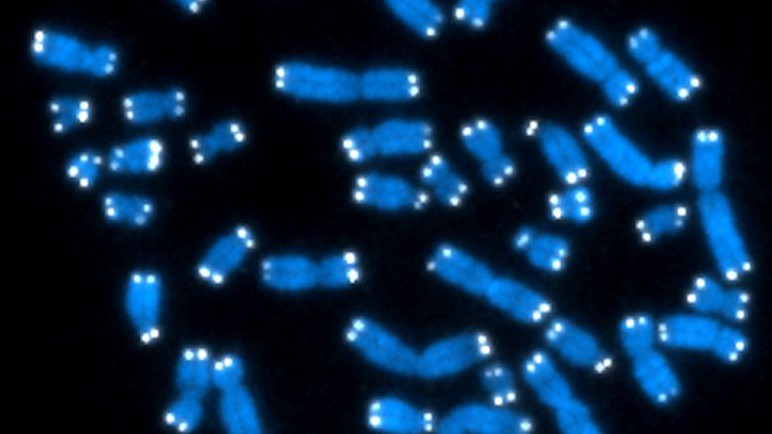
The telomeres can be seen as white dots on these chromosomes© National Institute of Health
In cancer cells, normal mechanisms governing the cellular life cycle have gone haywire. Cancer cells continue to divide indefinitely, without ever dying off, thus creating rapidly growing tumors. EPFL scientists have discovered a protein complex involved this deregulated process, and hope to be able to exploit it to stop tumor formation in its tracks.
All our cells come equipped with an automatic self-destruct mechanism; they are programmed to die after a certain number of divisions. This internal clock is of great interest to cancer researchers, because most forms of cancer exhibit a defect in this innate timing mechanism. Cancer cells continue to divide indefinitely, long past the moment at which a normal cell would self-destruct. A team of researchers from professor Joachim Lingner’s laboratory at EPFL has learned how this defect is regulated in a tumor. Post-doctoral researcher Liuh-Yow Chen led the team in publishing an article appearing in the journal Nature on the 4th of July 2012. Their hope is that the discovery will provide new targets for drug therapies to combat the deadly disease.
Cellular immortality, which is responsible for cancer formation, hearkens back to a critical function of the cells of the developing embryo. At the ends of every chromosome there is a special sequence of DNA known as a telomere, whose length is governed by the telomerase enzyme. This sequence represents the lifespan of the cell. Every time the cell divides, it is shortened, and when the telomere finally runs out, the cell dies. This reserve allows most cells to divide about 60 times – sufficient for the cell to play its given role in the organism, without succumbing to inevitable genetic mutation.
Cellular immortality, cancer’s common denominator
Normally, once the embryonic stage is completed, our cells stop producing telomerase – with the notable exception of somatic stem cells. But occasionally, a cell will mutate and reactivate production of the enzyme, so that when the cell divides, the telomere gets longer instead of shorter. This is what gives cancer cells their immortality.
“This mutation, on its own, is not enough to cause cancer,” explains Joachim Lingner, co-author and head of the lab. “But cellular immortality is a critical element in tumor formation in 90% of known cancers.” Researchers the world over hope to be able to stop the runaway growth of cancer cells by targeting this mechanism with drug therapy.
But interestingly enough, even in a cancer cell the telomere doesn’t grow indefinitely long. With each cell division it loses some 60 nucleotides, like most cells, but then the activated telomerase causes it to gain just as many back. The internal clock is reset to zero, and the cell becomes immortal. But there’s one interesting question here: What is stopping the telomere from getting indefinitely long?
Stopping the clock with three proteins
The EPFL team was able to provide an answer to this question; they identified three proteins that join together and then attach themselves to the telomere. A bit like a lid on a pot, this protein complex prevents telomerase from acting on the telomere. But in the cancer cell, their timing is off – their involvement takes place too late.
“If we could cause these proteins to act earlier, or if we could recreate a similar mechanism, the cancer cell would no longer be immortal,” explains Ligner. The cancer cells would die a normal death. Clinical applications are still a long way off, however, he insists. “Our discovery may allow us to identify potential targets - for example, a secondary protein to which these three proteins also attach. But right now our work is still in the basic research stages.”
- This research was conducted in the framework of the National Competence Centers in Research “Frontiers in Genetics”. The National Competence Centers are an initiative of the Swiss federal government with the goal to stimulate research and teaching in key sectors. http://www.frontiers-in-genetics.org