Decoding the liver's biological clock

A team of EPFL scientists has been able to observe the role of a circadian clock in controlling liver function for the first time, thanks to an ultra-high throughput DNA sequencing technique.
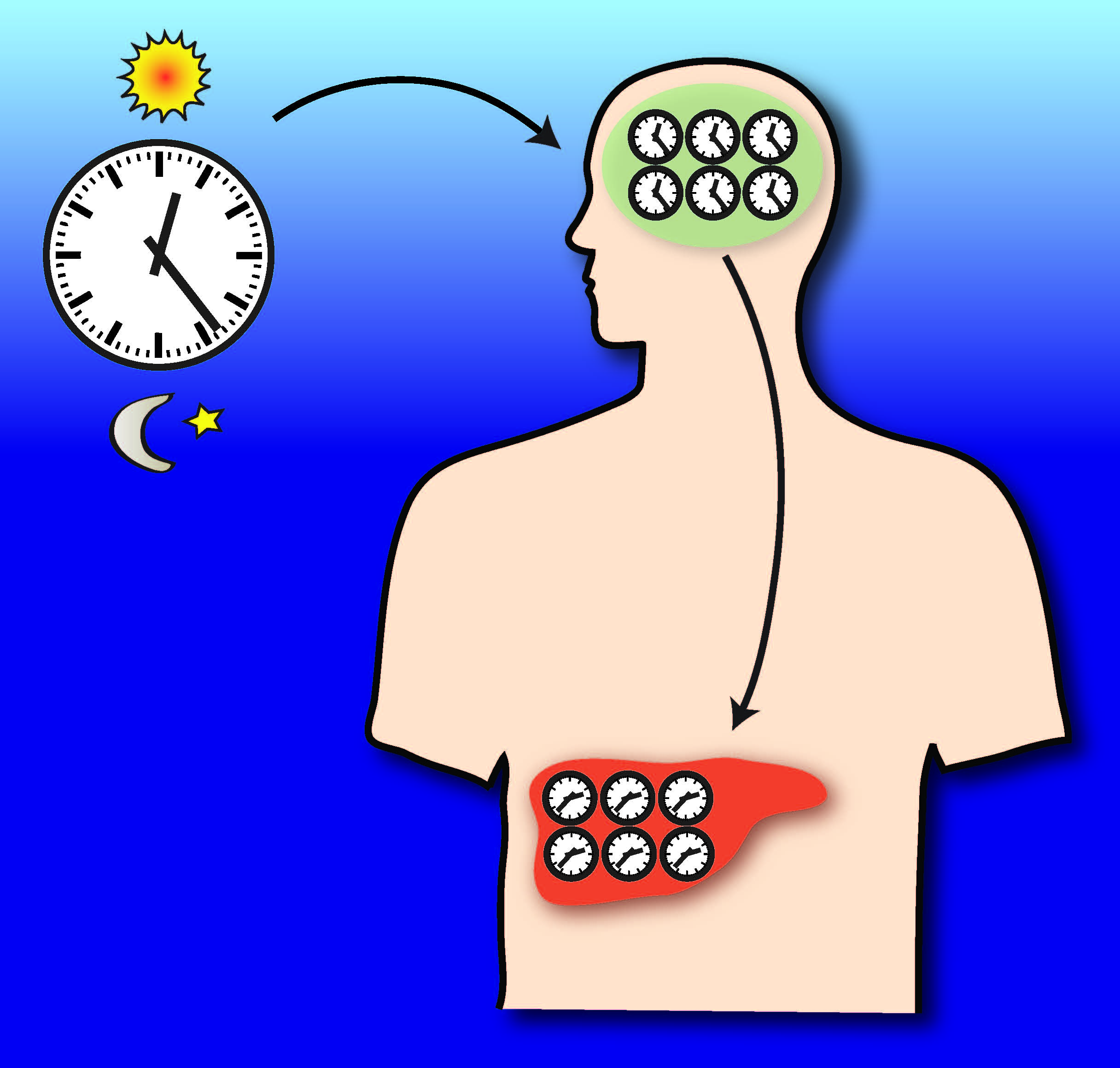
Most organisms evolved an internal molecular timekeeper known as the circadian clock. This clock acts as a genuine metronome that helps organisms coordinate their physiological and behavioral rythms with daily environmental cycles such as ligh-dark periods or temperature cycles. In mammals, these timekeepers modulate various physiological processes, such as cardiac rhythm, blood pressure, body temperature, hormone secretion, renal activity and digestive system activity on a daily basis. Jet lag, a well-known manifestation of these clocks, occurs when we put our internal clocks out of sync with the external diurnal cycle by jumping time zones on an airplane. It often takes several days to resynchronize the system. A team from EPFL’s Computational Systems Biology Lab, headed by Professor Felix Naef, has recently explored the role that circadian clocks play in controlling temporal aspects of liver function.
Guillaume Rey, a PhD student in the lab, used an ultra-high throughput DNA sequencing technique to characterize where and when a specific protein (BMAL1) physically interacted with DNA in the mouse liver. This protein is an important part of the so-called ‘core clock,’ and this was the first time the technique had been applied to circadian tissue. The results revealed information about physiological processes in the liver that are directly controlled by the core clock. “The unprecedented resolution of this technique shows us how our core clock modulates the temporal regulation of various metabolic functions that are active at different times of the day,” explains Naef.
As the liver can’t do everything simultaneously, it is advantageous to temporally separate incompatible biochemical processes. Results of the research indicate that the temporal distribution of metabolic functions satisfies the principle of resource optimization. For example, the clock helps regulating blood sugar levels over time. Absorbed sugar is first stored, then gradually released into the blood during the night, when we typically aren’t eating anything. Blood detoxification is also restricted to specific diurnal intervals. “We have gotten the first comprehensive picture of the way in which the liver clock relays temporal information to metabolic functions, such as carbohydrate metabolism or lipid biosynthesis,” notes Naef.
This discovery opens new avenues not only for metabolic research, but also in areas such as chronotherapy. By understanding how a biological clock like the liver clock works, it is possible to administer drugs based on biological rhythms, improving their effectiveness and limiting their undesired side effects. It has been shown, for example, that some drugs should be taken in the evening rather than in the morning. And in areas such as oncology, this could be used to help identify the most effective time for administering chemotherapy. Treatments could thus be optimized, adapted to the times of day when our bodies are the most receptive.
- The circadian clock -