COVID-19 test detects antibodies in hundreds of tiny blood samples
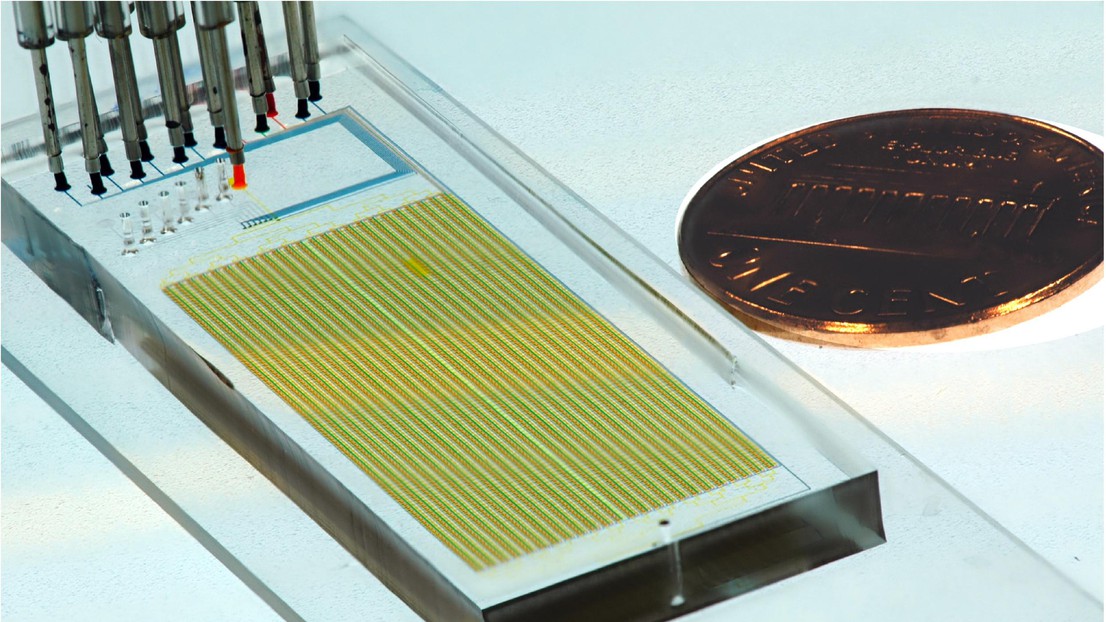
A MITOMI microfluidic device © Sebastian Maerkl / 2021 EPFL
Antibody testing can be a powerful tool for tracking the spread of SARS-CoV2 infections, the virus responsible for the COVID-19 pandemic. A group of scientists from EPFL, UNIGE and HUG have now developed a reliable and cheap antibody test that can analyze more than 1,000 samples at once and requires a small drop of blood, such as that from a finger prick.
After people get infected with the SARS-CoV-2 virus that causes COVID-19, they start to produce immune molecules called antibodies. COVID-19 antibody tests pick up on the presence of antibodies against SARS-CoV-2 in the blood. Because antibodies can take several days to weeks to develop, antibody tests can’t detect active infections, but they can help to find out what proportion of communities have been infected with the virus in the past. This knowledge is useful for epidemiological investigations, and informing public-health policies. Antibody tests are also a powerful tool to evaluate COVID-19 vaccine efficacy in clinical trials, when scientists look at the rise in antibodies after volunteers get a jab.
However, antibody tests rely on rather expensive reagents and typically require larger quantities of blood taken with a venous blood draw, which can only be performed by trained healthcare personnel. What’s more, some of the tests on the market are too inaccurate to deliver reliable results. Now, researchers from School of Engineering, UNIGE and HUG have developed a highly accurate test that can analyze hundreds of samples at the same time, using minute quantities of reagents and single drops of blood.
“The coolest thing about our approach is that you can do a lot of tests at once with minimal reagents, and you could even have people collect their own blood samples at home,” says study first author Zoe Swank, a former PhD student in the EPFL’s Laboratory of Biological Network Characterization led by Sebastian Maerkl.
In early 2020, Swank and Maerkl teamed up with Benjamin Meyer, a virologist at UNIGE Faculty of Medicine and scientific collaborator at HUG Division of Laboratory Medicine, and with Isabella Eckerle, a professor at UNIGE Faculty of Medicine and Medical Coordinator of the UNIGE-HUG Centre for Emerging Viral Diseases, and set out to repurpose a diagnostics platform that had been previously developed in Maerkl’s lab, so that it could be used to perform SARS-CoV-2 antibody tests.
The platform, which can analyze up to 1024 samples at once, consists of a complex network of tiny tubes carved into a plastic chip that is about the size of a USB stick. To perform the assay, the researchers feed individual blood samples and test reagents through the channels of this ‘microfluidic’ chip. If antibodies against SARS-CoV-2 are present in a blood sample, a molecule generates a signal that can be detected as a fluorescent glow under a microscope.
When the team tested blood samples from 155 individuals infected with SARS-CoV-2, the assay detected antibodies against the virus in 98% of cases. The assay is also extremely specific: it never detected antibodies against the virus in samples from people who had not been infected with SARS-CoV-2.
Because the microfluidic device is very small, the amounts of blood and reagents used are a fraction of those required for standard COVID-19 antibody tests. And running hundreds of assays on a single platform means that a person can perform more assays in less time, with potential cost savings on human labor, Maerkl says. “If you do a back-of-the-envelope calculation and take everything into consideration, including salary costs and the cost of reagents, it is about 0.5 Swiss francs per assay,” he says. “It’s almost negligible.”
To eliminate the need for collecting blood from people’s veins, Swank and her colleagues assessed whether they could use blood samples obtained from a finger prick — a simple procedure in which a finger is pierced with a tiny needle to obtain a small quantity of blood. The researchers tested three commercially available devices to perform finger-prick blood tests, including glucose test strips used by people with diabetes to measure their sugar blood levels.
The microfluidics-based antibody test could be successfully run on blood samples collected with all three methods, even when the blood was left to dry and stored for about one week at room temperature, or when samples were shipped by regular mail from Geneva to Lausanne. The study was published in PNAS.
“The approach of collecting blood in a decentralized way by a simple finger prick that can be even done at home, and a sophisticated laboratory-based assay with high diagnostic accuracy makes this test very attractive for large-scale epidemiological studies, explains Isabella Eckerle. It could even be used for remote geographic regions that lack sufficient laboratory capacity, for example to conduct seroprevalence studies in Sub-Saharan Africa.” She adds that “the small amount of blood and the collection by a finger prick, which is quick and almost painless, also makes this method very attractive for the use in children and offers a unique opportunity to assess seroprevalence rates in daycare centers or kindergartens.”
Maerkl and his collaborators are now using the test to determine the prevalence of antibodies against SARS-CoV-2 among kindergarteners in Geneva, in collaboration with Silvia Stringhini and Idris Guessous of the Population Epidemiology Unit at HUG. In the future, Maerkl says, this technology could make it possible for people to buy a blood sampling kit at a pharmacy or a supermarket, collect their own blood with a simple finger prick, and mail it to a central laboratory that analyzes the blood sample and returns the test results via email or a smart-phone app.
There’s no obvious limit on how many molecular assays can be done using the microfluidic diagnostics platform, Maerkl adds. “We’re interested in expanding this platform to other types of assays that would detect other biomarkers that people might want to measure — for example, blood ferritin levels in people with anemia,” he says.
A high-throughput microfluidic nanoimmunoassay for detecting anti–SARS-CoV-2 antibodies in serum or ultralow-volume blood samples
Zoe Swank, Grégoire Michielin, Hon Ming Yip, Patrick Cohen, Diego O. Andrey, Nicolas Vuilleumier, Laurent Kaiser, Isabella Eckerle, Benjamin Meyer, Sebastian J. Maerkl
Proceedings of the National Academy of Sciences May 2021, 118 (18) e2025289118; DOI: 10.1073/pnas.2025289118

