Combining research strategies with the needs of paralysis patients

© Jamani Caillet / EPFL
Focus on paralysis (2/1). Thanks to new treatment protocols developed by scientists at EPFL and CHUV, three patients with chronic paraplegia have been able to walk again. The protocols involve stimulating patients’ spinal cords with ultra-precise electrical impulses. In fact, several EPFL research teams are working on different ways to treat paralysis resulting from a stroke or spinal cord injury. How can their research strategies be combined to respond to patients’ needs?
 “Every step is important,” says Professor Stéphanie P. Lacour, head of EPFL’s Center for Neuroprosthetics (CNP). Her center develops treatments for patients who have become paralyzed following a stroke or spinal cord injury. While different research teams at EPFL are tackling this challenge from different angles, they all agree that the ultimate solution will involve personalized combinations of various forms of treatment.
“Every step is important,” says Professor Stéphanie P. Lacour, head of EPFL’s Center for Neuroprosthetics (CNP). Her center develops treatments for patients who have become paralyzed following a stroke or spinal cord injury. While different research teams at EPFL are tackling this challenge from different angles, they all agree that the ultimate solution will involve personalized combinations of various forms of treatment.
Replace
When a patient is no longer able to move a part of his body, one treatment option is to provide a device that can move it for him. Professor José del R. Millán, who holds the Defitech Foundation Chair in Brain-Machine Interfaces (BMIs), has developed BMIs that restore patients’ motor function in a primarily non-invasive manner. One of his more remarkable inventions is a BMI that enables patients to control a wheelchair with just their thoughts. Patients wear an electrode-equipped helmet that transmits their brain signals to a computer, which then executes the commands. His research team has used the same principle to develop a hand exoskeleton controlled by a patient’s motor cortex. This device not only gives patients back the use of their hands, it also provides sensory feedback on where their hands are and how they move.
In general, BMIs serve as a proxy for actual body movements, allowing paralyzed individuals to control physical devices with their minds and giving them greater independence. “The challenge is to correctly ‘read’ the movement that a patient wants to make, so that our systems can execute it,” says Millán.
Another option is to use a lower-limb exoskeleton to help paraplegics walk again. At EPFL’s Robotic Systems Laboratory (LSRO) headed by Professor Hannes Bleuler, scientists have been spending the past three years developing a walking-assistance device, called TWIICE. Made from carbon fiber and aluminum, TWIICE provides external hip and knee joints that enable patients to stand upright, walk and even climb up and down stairs – albeit with the help of crutches to ensure stability. The LSRO has just introduced a new version of the exoskeleton, called TWIICE One, that is lighter, more powerful and easier to use. And perhaps most importantly, its slimmer design means patients can put it on and take it off themselves.
Relearn
At EPFL’s Sion campus and Campus Biotech in Geneva, Friedhelm Hummel is performing cutting-edge research for the CNP and the Brain Mind Institute. Hummel is a world-renowned expert in helping patients regain functional use of their limbs after a stroke, in particular through the use of brain stimulation methods. His approach relates to the paralysis that results when specific areas of the brain are damaged; those areas are stimulated externally to help them process information properly and boost the efficacy of neurorehabilitation techniques, thereby restoring motor and cognitive function. However, Hummel’s main goal is to develop personalized high-precision treatments. “The damage done by a stroke can vary considerably from one patient to the next, especially in terms of the size of the lesion and the areas affected. And other patient-specific factors not necessarily related to the stroke itself can play a large role in the extent and process of recovery. We can’t take a one-size-fits-all approach to treatment,” he says.
To develop personalized stroke therapies, the first step is to understand the recovery process and the mechanisms involved. To that end, Hummel and three other CNP professors – Olaf Blanke, Silvestro Micera and Dimitri van de Ville – have been awarded a three-year, CHF 3 million grant from the ETH Board to carry out a cross-functional longitudinal study. The research team will use various methods including MRI, functional MRI, magnetic stimulation and EEG to identify the biomarkers involved and establish patient profiles. The results should enable doctors to deliver personalized brain stimulation protocols based on a patient’s exact condition, thereby allowing for maximum efficacy.
“Developing personalized therapies will also require pulling together the different kinds of neurotechnology available – such as brain stimulation, peripheral nerve stimulation and BMI-controlled exoskeletons – depending on what a patient needs,” says Hummel, who is working on a project of this kind supported by the Wyss Foundation.
The CNP professors plan to test a selection of different technologies to determine which ones work the best. “We find that patients stay more motivated if we continually adjust their rehabilitation protocols. That leads to greater plasticity and eventually better clinical results,” says Micera. Their methods could be used for partial cervical injuries as well.
Millán, who also works at Campus Biotech, is investigating a dual-therapy approach to enhancing motor function after a stroke. His approach entails using electrical impulses to stimulate the nerves of a paralyzed arm at precisely the same moment that the patient’s brain tries to carry out the movement. “The idea is to recreate the link between the two neural pathways where signals come in and are sent out,” says Millán. And his approach seems to work – EEGs on patients treated with this method clearly show an increase in the number of connections among the motor cortex regions in the damaged brain hemisphere, which means patients are able to carry out certain movements more easily.
Micera, who holds the Bertarelli Foundation Chair in Translational Neuroengineering, is working on a more invasive approach to help quadriplegics regain the ability to grasp objects with their hands, for example, by circumventing the damaged cells. In one of his studies, scientists implanted electrodes in a primate’s brain to record the signals generated when the primate wanted to grasp an object, while inducing temporary paralysis to prevent it from actually making the movement with its hand. When the scientists detected the brain signals to grasp an object, they artificially stimulated the nerves in the primate’s hand with electrodes. “The initial results are highly promising. We will carry out trials on additional primates, although it’ll take a few more years before we can clearly prove the method works. But the good news is that it can be quickly translated into clinical applications if it does. We have been using peripheral nerve stimulation on amputated patients for several years and cortical implants have already been approved by the FDA,” says Micera.
Repair
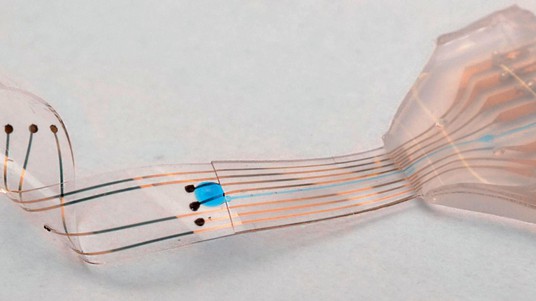
Returning a patient’s motor function to how it was before an injury or illness – insofar as possible – is the approach taken by other EPFL scientists including Lacour, who holds the Bertarelli Foundation Chair in Neuroprosthetic Technology, and Grégoire Courtine. The two are working together on an invasive method: neural implants that deliver electric impulses without affecting the mechanical properties of living tissue. In their method, a flexible neuroprosthetic is placed directly on a patient’s spinal cord, just above the lesion, and generates an electrical signal that does not come directly from the brain. Progress towards a clinical application of this type of research is very gradual; Lacour’s lab has just developed a miniaturized electronic platform that scientists can use to study peripheral nerve activity and devise new stimulation methods. The platform’s computer chips can be used to analyze the same type of neuronal tissue in vitro that exists in vivo.
Micera is also one of the pioneers working with Courtine on neural stimulation. “First we conducted joint research to better understand the mechanisms involved in stimulation, with the aim of being able to leverage them more fully. Then we then developed models allowing us to identify precisely which areas of a patient’s spinal cord are being stimulated so that we can regulate the stimulation. That is, instead of delivering constant impulses, we can change the amplitude, frequency or even location,” says Micera. The scientists have been able to obtain even better results by combining stimulation of the medulla oblongata with that of muscles and peripheral nerves.
Courtine’s lab, working in association with a team of US scientists, is also studying the regeneration of fully sectioned medulla nerve fibers. The research team has decoded the biological mechanisms required for the fibers to regenerate in complete lesions of the medulla, through experiments carried out successfully on mice and rats for the first time. They identified three elements necessary for the nerve fibers to grow: growth factors, proteins and hormones. Even if the process works just as well on humans, patients will still need to undergo physical therapy.
Regaining full use of their bodies
Restoring motor function is not the only objective of paralysis treatment – the cognitive aspect is important too. For instance, patients often suffer from phantom limb pain or a loss of feeling in their paralyzed limbs. Professor Blanke, who holds the Bertarelli Foundation Chair in Cognitive Neuroprosthetics, and his team have spent the past several years working on therapies to help restore bodily sensations through the use of various stimuli. Last year, they were able to give paraplegics feeling in their legs again using a virtual reality program and physical stimuli. Such methods can be useful in attenuating chronic pain. Electric stimulation of the kind that Micera is studying may be another way to restore bodily sensations. “We often find that stroke patients who have made significant progress in regaining motor function of a paralyzed limb still don’t use that limb because they’ve lost feeling in it,” says Micera.

Is treating paralysis one of the CNP’s key focus areas?
One of our strengths is sensorial-motor system research, as we bring together experts in complementary fields. Our research area requires a cross-disciplinary approach almost by definition, involving not only scientists but also doctors, physical therapists and bioengineers. Switzerland may be a small country with a relatively limited number of patients, but our center is home to a wide range of research methods and fields, making us fairly unique. That also makes it easier for us to work with local doctors and hospitals. The input of clinicians is essential because it helps us orient our research and determine which technologies to focus on.
Could the technology you develop be used more broadly?
Yes. Our innovations for paralysis treatment are readily transportable to other applications. For instance, the capacity to read and modulate neural information opens the door to new therapies and diagnostic methods for neurological disorders and other traumas. Our innovations draw on several of EPFL’s core competencies, from materials science and electronics to neuroscience, medicine and algorithm programming.
What’s the biggest challenge you face in your research?
Translating our innovations into clinical applications. That is, making the leap from mice to human beings. Of course, EPFL can’t do it alone; that’s why we work closely with accelerators like the Wyss Centre as well as businesses and clinics in the Lake Geneva region and beyond.