Barely porous organic cages for hydrogen isotope separation
© COSMO / 2019 EPFL
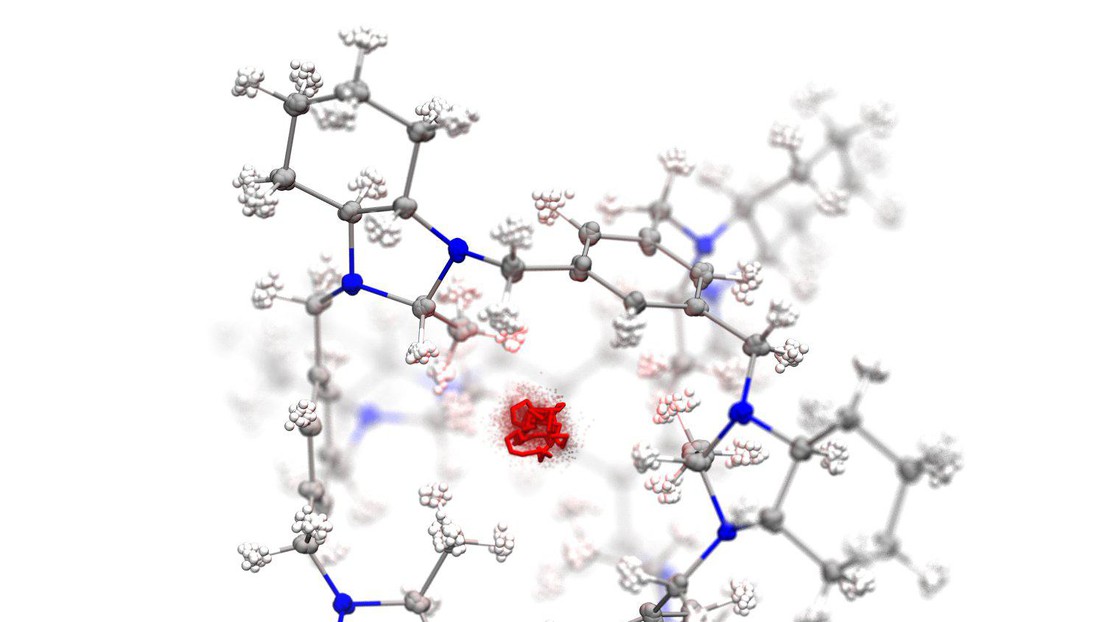
Porous materials obtained by assembling cage-shaped molecules of different types achieve high selectivity in separating hydrogen and deuterium gas by means of a quantum sieving process.
With the support of atomic-scale simulations performed at the Laboratory of Computational Science and Modeling, a team at the University of Liverpool has succeeded in synthesizing a material that makes it possible to separate efficiently H2 and D2. Ultra-pure deuterium is sought after as a non-radioactive tracer, and for applications to nuclear fusion. However, the separation process is highly challenging because of the chemical similarity of the two molecules, that only differ by the isotopic mass.
By synthesizing and assembling two different kinds of molecules, with carefully-tuned pore sizes, researchers were able to achieve simultaneously high selectivity and adsorption capacity. The mechanism underlying the separation, that relies on the quantum delocalization of the nuclei, can be simulated using sophisticated path integral molecular dynamics calculations, that were performed at EPFL.
The study has been published on Science.