Bacterial spears and spear-tips
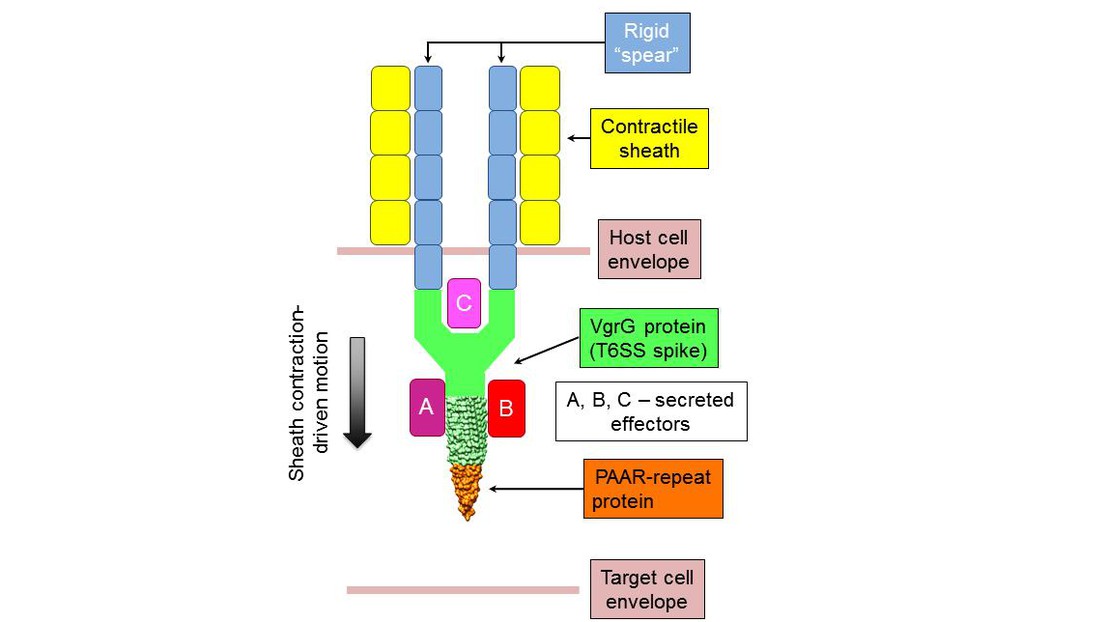
The T6SS spear structure © 2013 EPFL
Many bacteria use a spear-like structure to “stab” other cells and kill them. EPFL scientists have uncovered the structure of the poisonous tip of this “spear”, and have elucidated the way it works.
When they infect other cells, or when they want to defend themselves against attacking immune cells, many bacteria use multi-protein complexes called secretion systems. These act as tiny pipes that deliver toxic ‘effector’ proteins and DNA directly into the target cell, which can include anything from other bacteria to plant, animal and human cells, e.g. those of our immune system. The type VI secretion system (T6SS) is the most versatile of all the known secretion systems, but the way it selects effector proteins for delivery and how it delivers them into the target cell has always been a mystery. Publishing in Nature, scientists from EPFL and the Harvard Medical School were able to find answers to both questions.
Some of the world’s most devastating diseases, from pneumonia to cholera and tetanus to syphilis, are caused by bacterial infections. But most organisms have defense mechanisms to deal with invading bacteria; for example, the cells of our immune system attack unrecognized, potentially threatening micro-organisms. However, bacteria can respond to such defenses by using secretion systems: multi-protein complexes (i.e. complexes made up of many proteins) that allow bacteria to deliver toxic, ‘effector’ proteins into target cells and kill them.
One of these secretion systems, T6SS, is a widespread cytoplasmic organelle that looks like a microscopic spear with a spike-shaped protein complex at its tip. The spear is surrounded by a sheath that is assembled like a stretched-out spring. When a bacterium carrying T6SS is under stress (e.g. attacked by our immune cells), the spring-like sheath contracts, rapidly driving the spear out of the bacterium.
The team of Petr Leiman at EPFL, in collaboration with John Mekalanos from Harvard Medical School, has determined for the first time the complete protein composition of the T6SS spike and has demonstrated how it is tied to its function. By using X-ray crystallography, bioinformatics, and in vivo experiments on pathogenic bacteria, the researchers found that a new class of proteins called PAAR-repeat domains form a very sharp cone on the tip of the T6SS spike, just one atom in diameter. The authors suggest that this extremely sharp point is required for efficient piercing of the target cell’s membrane.
The researchers also discovered that PAAR-repeat domains can attach various toxic effectors to the spike of the spear, in a way ‘poisoning’ it. In addition, the flat base of the spear-tip binds to the T6SS spike, allowing different PAAR proteins to bind to it. Both these features make T6SS a truly versatile weapon.
This fascinating study shows that the toxic effector proteins are actually part of the spike, which is delivered into the target cell by means of a single, sheath contraction-driven and very rapid event (the entire process lasts only milliseconds). It also shows that the T6SS spike can be ‘poisoned’ with multiple effectors, each with a different toxic function. Petr Leiman says: “The new discovery by the Leiman and Mekalanos teams shows that T6SS is a remarkable microscopic machine that resembles both nature-evolved and man-made objects, from the venomous spine of a sea urchin to a ballistic missile with multiple independently targetable warheads (MIRV).”