Alzheimer's: Breaking the “code of Tau”
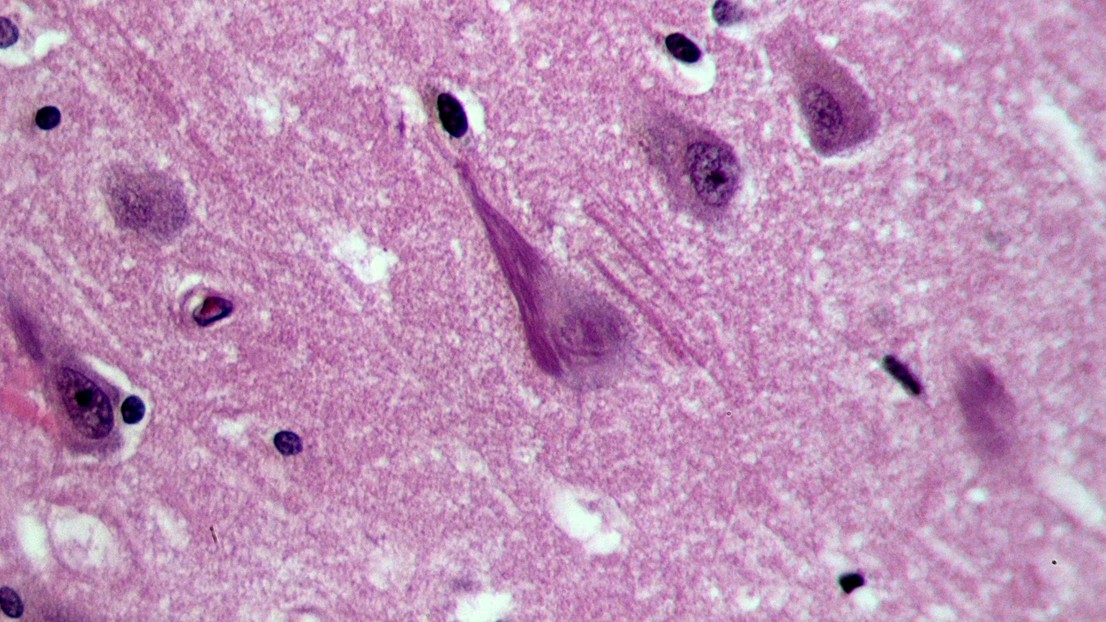
Neurofibrillary tangles (dark purple, elongated structure) in the hippocampus of an old person with Alzheimer-related pathology (credit: Wikimedia Commons user Patho)
Tau protein is one of the major targets in Alzheimer’s disease. EPFL scientists have now found a way to crack the previously inaccessible set of changes that turn Tau into a toxic molecule, known as the “code of Tau”.
In Alzheimer’s disease, a protein called Tau, aggregates into twisted fibrils that produce characteristic tangles inside neurons. The tangles cause the neurons’ transport systems disintegrate, disrupting the movement of essential nutrients and finally killing the cells. The brain’s functions are then affected and produce the disease’s symptoms.
Given its central role in the pathology of Alzheimer’s and other neurodegenerative diseases called “tauopathies” (e.g. Pick’s disease), Tau has become one of the most actively pursued therapeutic targets in both academic and commercial research. But one of the major challenges is the ability to isolate and study the numerous post-translational modifications (PTMs) that Tau undergoes before it becomes toxic.
This so-called “code of Tau” is now about to be cracked. In a paper published in JACS, Hilal Lashuel and Mahmood Haj-Yahya report a new method that allows scientists to introduce specific PTMs into Tau and then study their effects. The method is published in the Journal of the American Chemical Society.
The method is based on building proteins from polypeptide fragments that are either produced chemically or expressed in cells and then assembled sequentially in a test tube, almost like Legos. In this case, the scientists were able to produce Tau protein (441 amino acids), from five fragments.
This “semisynthetic” strategy offers great flexibility for incorporating one or several modified amino acids at the desired sites in each building block. The method also preserves the native sequence of Tau, including parts that which have recently been shown to play critical roles in its disease-related processing.
To demonstrate the flexibility of their methods, the scientists produced variants of Tau with different types of PTMs in different parts of the protein. “Our semisynthetic strategies not only allow systematic investigation of site-specific post-translational modifications, but enable for the first time the investigation of the cross-talk between different modifications in the most important regions of the protein which is implicated in regulating Tau aggregation and functional properties,” says Mahmood Haj-Yaha, the postdoc who led the project.
Lashuel’s lab now plans to use this approach to build a library of Tau that represents the diversity of normal and pathological versions of the protein that have been detected in the brain and biological fluids. They will then use a battery of biochemical and biological assays to determine which of these modifications inhibit or exacerbate Tau aggregation and toxicity to identify new therapeutic targets and pathways for the treatment of Alzheimer’s disease.
“These advances enable us for the first time to systematically investigate the role of PTMs in regulating the misfolding, aggregation, toxicity and Tau pathology spreading in the brain,” says Hilal Lashuel. “It is also a unique opportunity to advance biomarker discovery by facilitating the development of more accurate assays for detecting and measuring different Tau species during the progression of Alzheimer’s and related tauopathies.”
École polytechnique fédérale de Lausanne (EPFL)
Mahmood Haj-Yahya, Hilal A. Lashuel. Protein semisynthesis provides access to Tau disease-associated post-translational modifications (PTMs) and paves the way to deciphering the Tau PTM code in health and diseased states. J. Am. Chem. Soc. 23 April 2018. DOI: 10.1021/jacs.8b02668