AI to rank the synthesizability of materials for carbon capture
© 2022 EPFL
EPFL researchers, together with colleagues in Paris and the U.S., have applied artificial intelligence (AI) to a long-standing problem in materials science – identifying which structures within massive computer-generated databases are good candidates for actual fabrication. NCCR MARVEL’S Michele Ceriotti, professor at the School of Engineering, and coworkers focused their study on hypothetical zeolites, materials that show promise for capturing carbon dioxide emissions, as well as for catalysts for green chemistry.
Zeolites are nanoporous crystals that have been utilized for more than six decades in a number of industrial processes, particularly in refining petroleum and separating chemical mixtures. While much effort has been put into identifying and synthesizing new zeolites for modern needs such as producing clean biofuels and capturing carbon dioxide, success has been largely theoretical. While massive databases of hypothetical zeolites have been generated containing millions of new framework structures, none has been made in the lab.
“This problem, which is known as the ‘zeolite conundrum,’ has severely limited the pace of the clean energy transition,” said Michele Ceriotti. “Finding the few hypothetical zeolites that can actually be synthesized in the lab is like finding a needle in a gigantic haystack.”
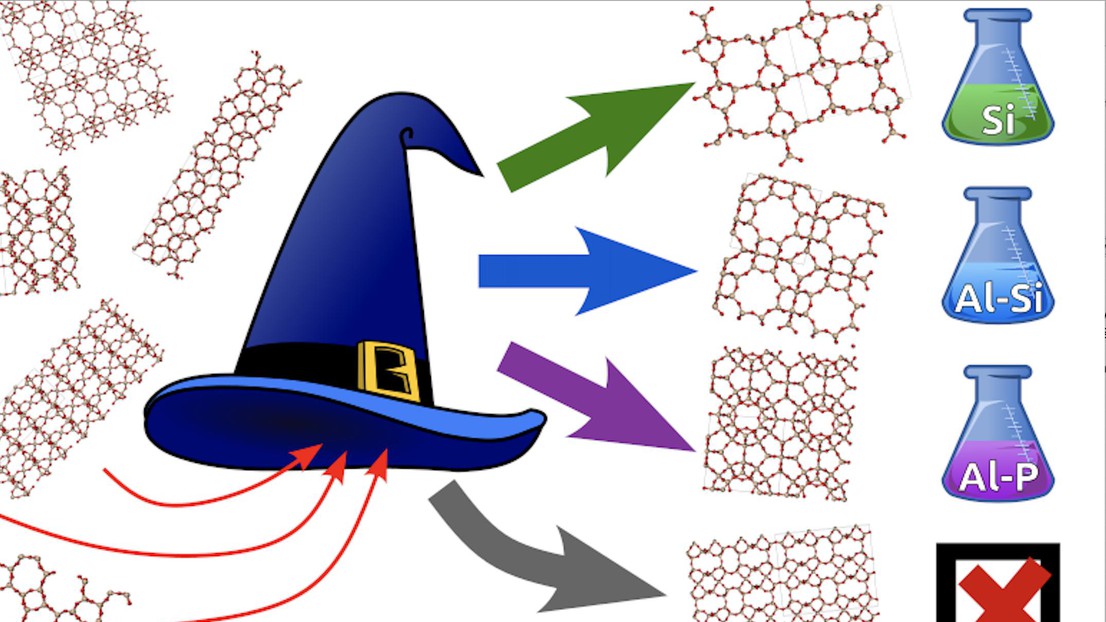
Ceriotti and coworkers – Benjamin Helfrecht at EPFL, professor Rocio Semino and Giovanni Pireddu at the Sorbonne University in Paris and University of Massachusetts chemistry professor Scott Auerbach – developed an algorithm called the “sorting hat” that uses physically-inspired machine learning techniques to distinguish between the 255 already-synthesized zeolites and the more than 300,000 hypothetical framework structures, pre-selected for their (theoretical) stability. They created a short list of hypothetical zeolites that are so similar to real ones that they are “misclassified” by the sorting hat as real materials, making them good candidates for actual synthesis.
After filtering their results by additional criteria, including the potential for stabilizing them during synthesis, the researchers proposed three leading hypothetical candidates for synthesis. Their analysis also categorized real zeolites into four compositional classes or “houses.” This partitioning into houses allowed the researchers to propose a chemical composition to pursue in the laboratory for making the hypothetical zeolites – the first step in determining a recipe for synthesis.
“As is the case for many synthetic tasks, making zeolites is a form of art, guided by experience, chemical intuition and serendipity,” the researchers said. “The zeolite sorting hat introduces data-driven techniques and rational design into the process of selecting candidates that we hope will accelerate the rate of discovery that, in turn, will improve the predictive capabilities of the model in a positive feedback mechanism that will progressively take the guesswork out of zeolite synthesis.”
The study was published this week in the journal Digital Discovery.