A secret language of cells? New cell computations uncovered
© 2022 EPFL
Throughout evolution, individual cells have been making successful decisions on their own, even while forming parts of vast networks, such as neurons and glia in the human brain. Now scientists from the EPFL Blue Brain Project and King Abdullah University of Science and Technology (KAUST) have published a new theory describing a secret language that cells may use for internal dialog about the external world.
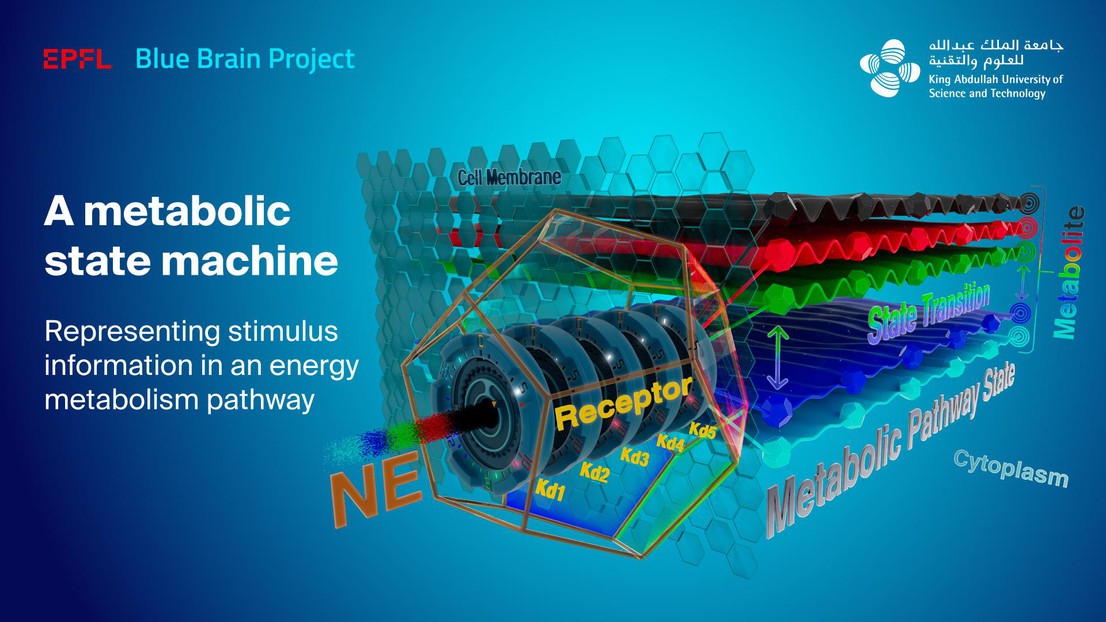
Using a computational model, they hypothesize that metabolic pathways, which are primarily a means of extracting energy and building block molecules from glucose and other substrates to feed the brain, might also be capable of coding details about neuromodulators that stimulate increases in energy consumption. If true, this would open the door to a nearly infinite number of possibilities for information processing in nervous systems as component cells could compute in previously unexplored ways. Such a mechanism would also help explain the remarkable energy efficiency of brains.
The aim of the Blue Brain Project is to establish simulation neuroscience as a complementary approach to understanding the brain, alongside experimental, theoretical and clinical neuroscience, by building the world’s first biologically detailed digital reconstructions and simulations of the mouse brain. In a study recently published in the Journal of Theoretical Biology, the collaboration with KAUST demonstrated how two of these pillars – theory and simulation - can work in tandem using a model of astrocytic energy metabolism. The authors confirmed the plausibility that this type of pathway might be capable of coding information about stimuli in addition to its known functions in cellular energy and carbon budgets. Considering how many metabolic pathways are active simultaneously, these mechanisms could significantly increase the computational capabilities of neurons by giving them an expanded tool set for adaptation and decision-making.
The flux of matter through these pathways involves handing-off metabolite-products from one enzyme-catalyzed reaction to the next in the chain. The results in this study suggest that these pathways can also quantitatively capture and transmit various characteristics about stimuli, including intensity and duration features of waves of neuromodulators arriving at the cell surface. The mechanism involves considering the entire chain of events from neuromodulator receptor activation to energy metabolite production as an excitable unit or metabolic state machine (Coggan et al., 2020).
“From this,” explains Lead Author, Blue Brain’s Jay S. Coggan, “we could show how a metabolic pathway can translate external stimuli into production profiles of energy-carrying molecules such as lactate with a precision beyond simple signal transduction or amplification. Such metabolic pathways, and possibly other kinds of coupled enzymatic reactions, might be well-positioned to code an additional level of salient information about a cell’s environmental demands. This hypothesis has implications for the computational power and energy efficiency of the brain,” he concludes.
Single cells could still have some undiscovered tricks
“The teams’ simulations of neuromodulator-stimulated glucose metabolism in an astrocyte suggest that metabolic pathways could be capable of more information processing than we previously realized,” explains KAUST’s Prof. Pierre Magistretti. “These systems exhibit concentration-dependent responses with state changes similar to the more familiar action potentials that can capture and transmit signal properties from ligand-receptor binding through controllable metabolite fluxes. Despite everything that is already known about how single-cells think or respond to their environment, they likely still have some undiscovered tricks.”
--------------------------
Citation
Coggan, J. S., Keller, D., Markram, H., Schürmann, F., & Magistretti, P. J. (2022). Representing stimulus information in an energy metabolism pathway. Journal of Theoretical Biology, 540, 111090. https://doi.org/10.1016/j.jtbi.2022.111090
This study was supported by a CRG grant from King Abdullah University of Science and Technology "KAUST-EPFL Alliance for Integrative Modeling of Brain Energy Metabolism" [OSR-2017-CRG6-3438] (PJM); the Blue Brain Project, a research center of the École Polytechnique Fédérale de Lausanne, from the Swiss government's ETH Board of the Swiss Federal Institutes of Technology (HM); and NCCR Synapsy (PJM).