Visualizing chemical reactions
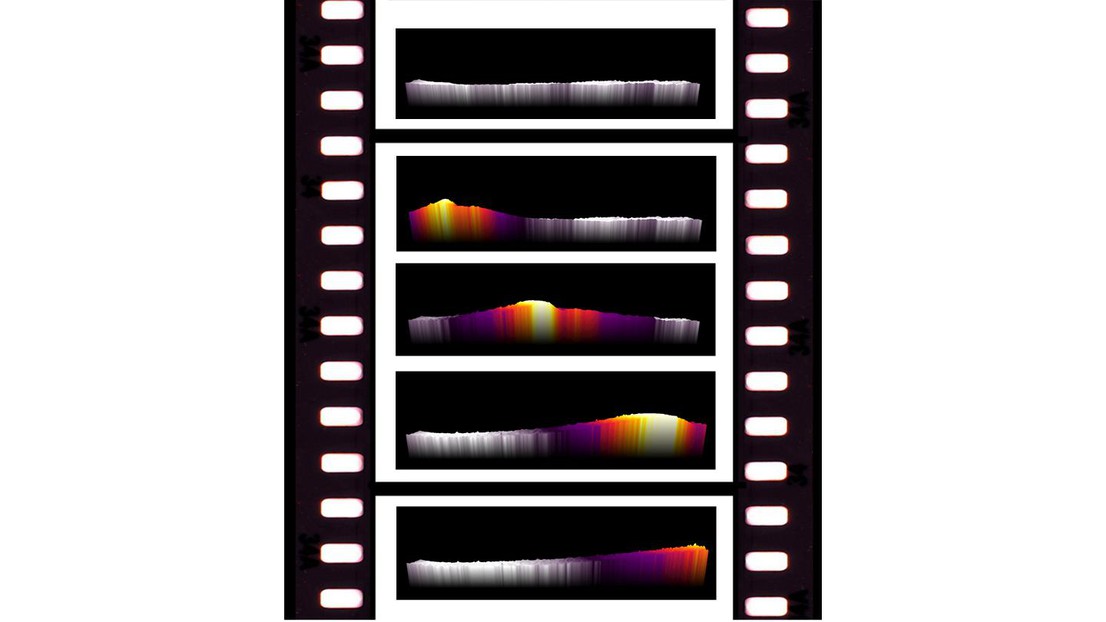
Thermal image sequence showing the exothermic surface reaction front moving over the catalyst. Source: ACS Catalysis;
Researchers at EPFL have developed a reactor system and an analysis method that has allowed them to observe the real-time production of synthetic natural gas from CO2 and H2 for the first time.
Infrared (IR) thermography is used to determine the temperature of humans and objects with high precision and without interfering with the system. A single image taken with an IR camera can capture the same amount of information as hundreds to millions of thermocouples (temperature sensors) at once. Furthermore, modern IR cameras can achieve fast acquisition frequencies of over 50 Hz, which allows the investigation of dynamic phenomena with high resolution.
Now, scientists at EPFL have designed a reactor that can use IR thermography to visualize dynamic surface reactions and correlate it with other rapid gas analysis methods to obtain a holistic understanding of the reaction in rapidly changing conditions. The research was led by Robin Mutschler and Emanuele Moioli at the lab of Andreas Züttel (EPFL and Empa) and they collaborated with researchers at the Polytechnic University of Milan.
The scientists applied their method to catalytic surface reactions between carbon dioxide and hydrogen, including the Sabatier reaction, which can be used to produce synthetic methane from renewable energy by combining CO2 from the atmosphere and H2 from water splitting, thus enabling the synthesis of renewable synthetic fuels with similar properties to their fossil counterparts which is why the Sabatier reaction has attracted a lot of attention recently. A catalyst is required in the Sabatier reaction to activate the relatively inert CO2 as a reactant.
In particular the EPFL researchers focused on the investigation of dynamic reaction phenomena occurring during the reaction activation from different initial catalyst states.
“The reaction on the catalyst is favored by a hydrogenated surface while an exposure to CO2 poisons the catalyst and inhibits a fast reaction activation,” says Mutschler.
“Thanks to this new approach, we could visualize new dynamic reaction phenomena never observed before,” says Moioli.
In their work they showed the catalyst working and responding to the changes in the feed gas composition and during its activation from different initial states in real time for the first time. By means of their results, the reaction startup and activation behavior are now better understood and it can lead to optimized reactor and catalyst designs to improve the performance of these reactor systems working in dynamic conditions.
This is crucial since renewable energy typically provides energy and reactants stochastically and therefore the reactors converting renewable energy to fuels have to be adapted to work in dynamic conditions under certain circumstances.
Other contributors
Empa Materials Science & Technology
Swiss National Science Foundation (SNSF)
Robin Mutschler, Emanuele Moioli, Kun Zhao, Loris Lombardo, Emad Oveisi, Alessandro Porta, Leonardo Falbo, Carlo Giorgio Visconti, Luca Lietti, and Andreas Züttel. Imaging catalysis: Operando investigation of the CO2 hydrogenation reaction dynamics by means of infrared thermography. ACS Catalysis. DOI: 10.1021/acscatal.9b04475