Targeting blood vessels to improve cancer immunotherapy
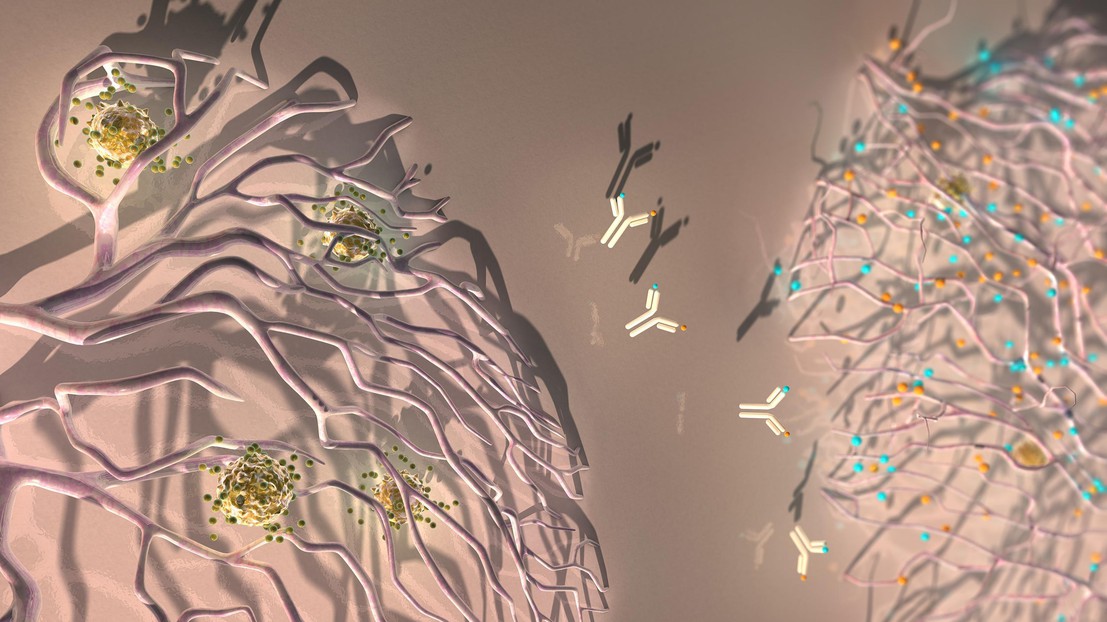
An antibody against ANGPT2 and VEGFA reprograms the tumor blood vessels to facilitate the extravasation of anti-tumoral T cells. Credit: Ella Maru Studio
EPFL scientists have improved the efficacy of cancer immunotherapy by blocking two proteins that regulate the growth of tumor blood vessels.
Cancer immunotherapy aims to enhance or restore the ability of the patient’s immune system – namely T cells – to recognize and attack cancer. But tumors use several strategies to fight back immune attacks, making immunotherapy efficacious only in a minority of the patients. For example, they produce blood vessels that block, rather than facilitate, the arrival of T cells. EPFL scientists have now improved the efficacy of immunotherapy against different cancer types by reprogramming the tumor blood vessels. The study is published in Science Translational Medicine, and is featured on the cover of the journal.
Starving tumors
Tumor blood vessels are essential for providing oxygen and nutrients to the growing cancer cells. The laboratory of Michele De Palma at EPFL focused on two proteins, called VEGFA and ANGPT2, which tumors produce to stimulate the growth of new blood vessels. Blocking the actions of VEGFA and ANGPT2 may curb the growth of the blood vessels, limit the provision of oxygen and nutrients, and starve the tumors.
To block both VEGFA and ANGPT2, the EPFL scientists used an antibody called A2V. The team tested A2V in experimental models of breast cancer, pancreatic cancer, and melanoma. They found that A2V provides clear therapeutic benefits, whereas antibodies that block either VEGFA or ANGPT2 alone had more limited efficacy. Importantly, A2V also inhibits metastasis, a condition that is frequently lethal in patients with cancer.
A2V reprograms the tumor blood vessels
Under the influence of VEGFA and ANGPT2, the tumor blood vessels also acquire an aberrant structure that impedes the passaging of T cells, thus limiting the efficacy of immunotherapy. A2V caused the regression of many tumor blood vessels, but some persisted after the therapy.
“One interesting finding was that A2V not only regressed most of the tumor blood vessels, but also reversed the aberrant features of those that had remained, making them similar to normal blood vessels and more permissive to the arrival of anti-tumoral T cells”, says De Palma. Indeed, A2V promoted the “extravasation” of activated T cells into the tumors, a process that is required to initiate an immune response against the tumor.
A2V helps checkpoint blockers
Tumors can evade detection and attacks by patrolling immune cells, such as T cells. Tumors accomplish this by expressing certain proteins, called “immune checkpoint ligands”. One of these is the protein PD-L1 (programmed death ligand 1), which binds a receptor (PD-1) that is present on the surface of T cells, stopping them from attacking the tumor. A way to circumvent this problem is to use drugs called checkpoint inhibitors. These are usually antibodies that find and bind the immune checkpoint proteins on tumors, thus leaving them open to immune attacks.
The EPFL researchers found that the accumulation of activated T cells around the tumor blood vessels, which was promoted by A2V therapy, also triggered a defensive response: the blood vessels started to produce the checkpoint ligand PD-L1 in an effort to “blind-sight” the attacking T cells. However, the researchers found that it is possible to overcome this setback by blocking the PD-1 receptor. Indeed, an anti-PD-1 antibody further improved the anti-tumoral effects of A2V.
“These data remind us that mechanisms of resistance to anti-cancer therapies are always beyond the corner. While A2V normalized the tumor blood vessels and facilitated the arrival of activated T cells, the anti-tumoral T cells became rapidly suppressed upon their extravasation to the tumor microenvironment”, says De Palma.
The study has important implications for cancer immunotherapy. “Our work suggests that certain anti-angiogenic drugs, namely ANGPT2 inhibitors, have more profound effects on tumors than initially thought. Besides targeting the blood vessels, they also help initiate anti-tumoral immune responses, which can be reinforced by immune checkpoint blockade”.
This work was mainly funded by the Leenaards Foundation, the Swiss Cancer League, the San Salvatore Foundation, and Roche. It also included contributions from the Roche Innovation Centers at Basel and Munich.
Reference
Martina Schmittnaegel, Nicolò Rigamonti, Ece Kadioglu, Antonino Cassará, Céline Wyser Rmili, Anna Kiialainen, Yvonne Kienast, Hans-Joachim Mueller, Chia-Huey Ooi, Damya Laoui, Michele De Palma. Dual angiopoietin-2 and VEGFA inhibition elicits anti-tumor immunity that is enhanced by PD-1 checkpoint blockade.Sci. Transl. Med. 9, eaak9670, 12 April 2017. DOI: 10.1126/scitranslmed.aak9670