Restriction Enzyme Analysis of Double-Stranded DNA on Carbon Nanotube
© 2018 ACS Publications
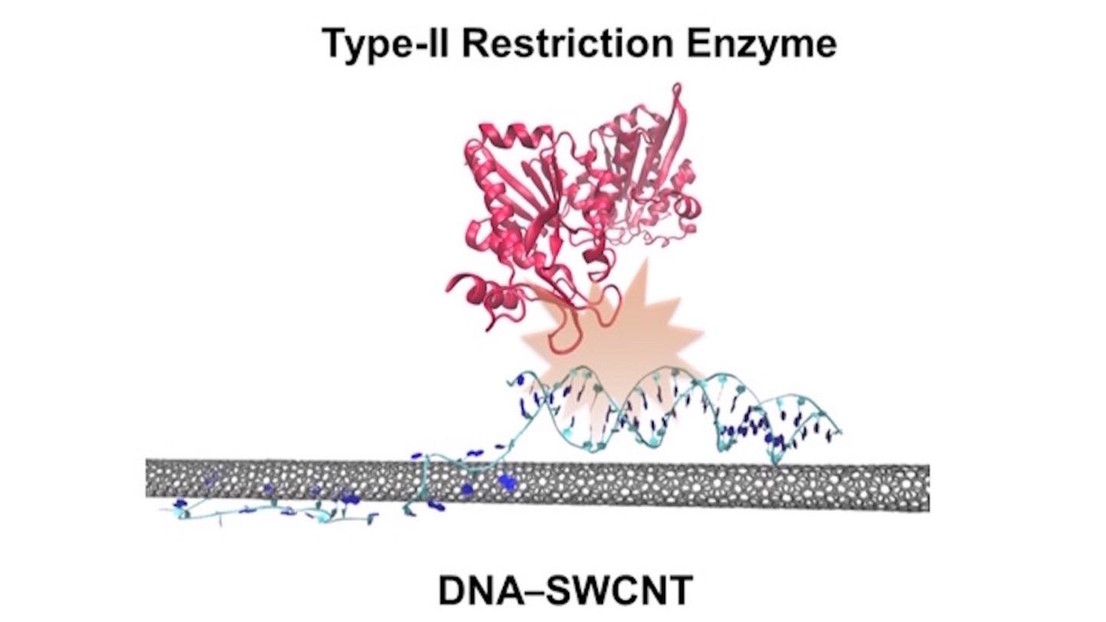
Shang-Jung Wu and coauthors publish the article "Restriction Enzyme Analysis of Double-Stranded DNA on Pristine Single-Walled Carbon Nanotubes" in ACS Applied Materials and Interfaces.
Nanoprobes such as single-walled carbon nanotubes (SWCNTs) are capable of label-free detection that benets from intrinsic and photostable near-infrared uorescence. Despite the growing number of SWCNT-based applications, uncertainty surrounding the nature of double-stranded DNA (dsDNA) immobilization on pristine SWCNTs has limited their use as optical sensors for probing DNA−protein interactions. To address this limitation, we study enzyme activity on unmodied dsDNA strands immobilized on pristine SWCNTs. Restriction enzyme activity on various dsDNA sequences was used to verify the retention of the dsDNA’s native conformation on the nanotube surface and to quantitatively compare the degree of dsDNA accessibility. We report a 2.8-fold enhancement in initial enzyme activity in the presence of surfactants. Förster resonance electron transfer (FRET) analysis attributes this enhancement to increased dsDNA displacement from the SWCNT surface. Furthermore, the accessibility of native dsDNA was found to vary with DNA conguration and the spacing between the restriction site and the nanotube surface, with a minimum spacing of four base pairs (bp) from the anchoring site needed to preserve enzyme activity. Molecular dynamics (MD) simulations verify that the anchored dsDNA remains within the vicinity of the SWCNT, revealing an unprecedented bimodal displacement of the bp nearest to SWCNT surface. Together, these ndings illustrate the successful immobilization of native dsDNA on pristine SWCNTs, oering a new near-infrared platform for exploring vital DNA processes.
The authors are thankful for support from the Swiss National Science Foundation (SNSF) Assistant Professor (AP) Energy Grant.