Mirror images and complex molecules
© 2013 EPFL
EPFL scientists have synthesized scaffolds for potential anti-cancer and anti-dementia drugs from simple and cheap starting materials.
Carbon-hydrogen (C–H) bonds are at the heart of most organic compounds. They have been of tremendous scientific interest, as cleaving the bond (C–H functionalization) offers a powerful tool for building complex molecules from widely available starting materials. Although for many years chemists believed that C-H bonds were unreactive, a number of impressive C-H functionalization reactions have now appeared. However, only few of these reactions can distinguish between enantiomers of molecules and allow choosing one enantiomer over another. In a recent Angewandte Chemie publication, EPFL scientists describe a method that allows the enantioselective production of a compound class, the dibenzazepinones, bearing some potential for Alzheimer’s disease drugs.
In chemistry, an enantiomer is a molecule that is the mirror image of another one – much like our hands, the two molecules cannot be superimposed. This phenomenon is called chirality, and is widespread in nature. And since enantiomers have equal energies, they are produced in equal amounts in a reaction. But despite having similar structures and atoms, enantiomers can have entirely different properties, simply because of their different chiralities. And that can cause problems when only one of the two is required.
Many biological building blocks in nature, like sugars and amino acids, are produced exclusively as one enantiomer. Consequently, biological systems possess a high degree of chemical chirality and will often react differently when exposed to enantiomers of a compound. Examples of this enantioselectivity include, flavor, odor, drug effectiveness and drug safety. And given the importance of enantiomers, reactions that can be enantioselective have been a focus of much research activity; nevertheless, they are notoriously difficult to achieve.
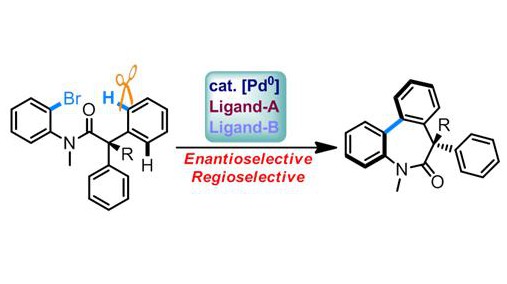
The group of Nicolai Cramer at EPFL succeeded in achieving C–H functionalization – cleaving the C–H bond – with a high rate of enantioselectivity. The team has a longstanding interest in C–H functionalizations for creating chiral nitrogen-containing heterocycles, which are ubiquitous in natural products and bioactive compounds. Drawing from this, they focused on designing a reaction that could produce dibenzazepinones, a highly important class of compounds that are used in potential drugs against cancer and Alzheimer’s disease.
The method involved the cyclization of two aryl groups of the substrate by a C–H bonds cleavage. The researchers used palladium complexes as catalysts for the reaction, as such transition metal-catalyzed C–H transformations have shown much success in the field. Their strategy was based on the cooperative effects between a chiral phosphine ligand and a bulky carboxylate that occurred during what is referred to as the concerted deprotonation-metalation (CMD) step, which has emerged as efficient tactic to functionalize C-H bonds.
The result was the synthesis highly functionalized and relevant dibenzazepinones and doing so with excellent ability to select the ‘correct’ enantiomers. With these substrates, the researchers have found a way to complete selectivity over competing C–H activations, which could also produce compounds with five- or six-membered rings. In addition, it is noteworthy that the process uses cheap and widely available materials as starting material. The breakthrough is expected to have great application in the future of rapid and modular chemical assembling of complex molecules on an industrial scale.