Computing the Free Energy of Defects
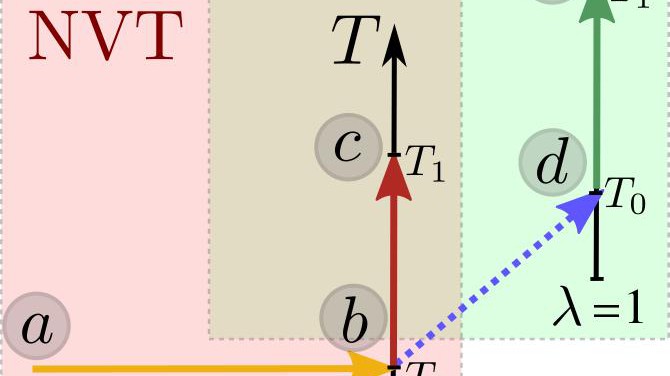
Using thermodynamic integration to compute free energies © 2018 EPFL
Entropy is a measure of disorder, and it does indeed play an important role in determining the stability of defects. Research by Bingqing Cheng, from the Laboratory of Computational Science and Modelling, shows how this oft-neglected term affects the stability of lattice defects in metals.
The Gibbs free energy is the fundamental thermodynamic potential underlying the relative stability of different states of matter under constant-pressure conditions. However, computing this quantity from atomic-scale simulations is far from trivial. As a consequence, all too often the potential energy of the system is used as a proxy, overlooking entropic and anharmonic effects. Here we discuss a combination of different thermodynamic integration routes to obtain the absolute Gibbs free energy of a solid system starting from a harmonic reference state. This approach enables the direct comparison between the free energy of different structures, circumventing the need to sample the transition paths between them. A paper by Bingqing Cheng, from the Laboratory of Computational Science and Modelling, showcases this thermodynamic integration scheme by computing the Gibbs free energy associated with a vacancy in BCC iron, and the intrinsic stacking fault free energy of nickel. These examples highlight the pitfalls of estimating the free energy of crystallographic defects only using the minimum potential energy, which overestimates the vacancy free energy by 60% and the stacking-fault energy by almost 300% at temperatures close to the melting point.