Benzothiazinones
Benzothiazinones
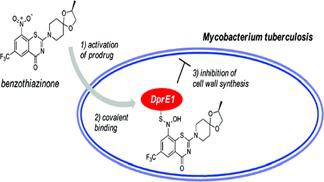
people.epfl.ch/stewart.coleBenzothiazinones: Prodrugs That Covalently Modify the Decaprenylphosphoryl-β-D-ribose 2′-epimerase DprE1 of Mycobacterium tuberculosis.
Benzothiazinones (BTZs) form a new class of potent antimycobacterial agents. Although the target of BTZs has been identified as decaprenylphosphoryl-β-d-ribose 2′-epimerase (DprE1), their detailed mechanism of action remains obscure. The groups of Profs. Kai Johnsson (LIP - Laboratory of Protein Engineering) and Stewart Cole (Chair of Microbial Pathogenesis) demonstrate that BTZs are activated in the bacterium by reduction of an essential nitro group to a nitroso derivative, which then specifically reacts with a cysteine residue in the active site of DprE1.
Trefzer C. et al., J. Am. Chem. Soc. ASAP, DOI: 10.1021/ja106357w (2010)